A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The dipole moment of is 1.5 D. The dipole moment of is
Text Solution
|
- The dipole moment of is 1.5D what will be the dipole moment of
Text Solution
|
- The dipole moment of chlorobenzene is 1.73 D . The dipole moment of p-...
Text Solution
|
- If the dipole moment of Toluene and Nitro - benzene are 0.43 D and 3.9...
Text Solution
|
- Dipole Moment,determination of shape based on dipole oment,magnitude o...
Text Solution
|
- If the dipole moment of Toluene and Nitro - benzene are 0.43 D and 3.9...
Text Solution
|
- Dipole moment of is 1.1 D hence dipole moment of given compound will ...
Text Solution
|
- Dipole momentof is 1.5 D. The dipole moment of is
Text Solution
|
- Define dipole moment. Give the mathematical expression of dipole momen...
Text Solution
|
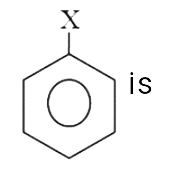 is 1.5 D.
is 1.5 D.