A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
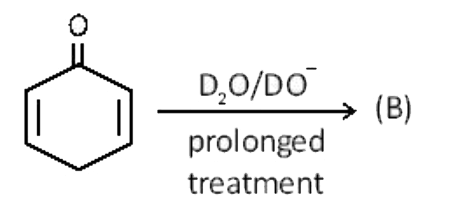
- After prolonged treatement of (A) by D(2)O//DO^(-), the maximum possib...
Text Solution
|
- What will be the number of hydrogen atoms (Y) replaced by D on prolong...
Text Solution
|
- After prolonged treatment of (A) by D(2)O"//DO^(-), the difference in ...
Text Solution
|
- Empirical formula of compound is CH(2)O .If its molecular weight is 18...
Text Solution
|
- Empirical formula of compound is CH(2)O.If its molecular weight is 180...
Text Solution
|
- After prolonged treatement of (A) by D(2)O // DO^(-) , the maximum pos...
Text Solution
|
- Temperature at which the density of D(2)O is maximum is-
Text Solution
|
- The empirical formula of a compound is CH(2)O . Its molecular weight i...
Text Solution
|
- A sample of water contains x % of D(2)O. Its molecular weight is 19. T...
Text Solution
|