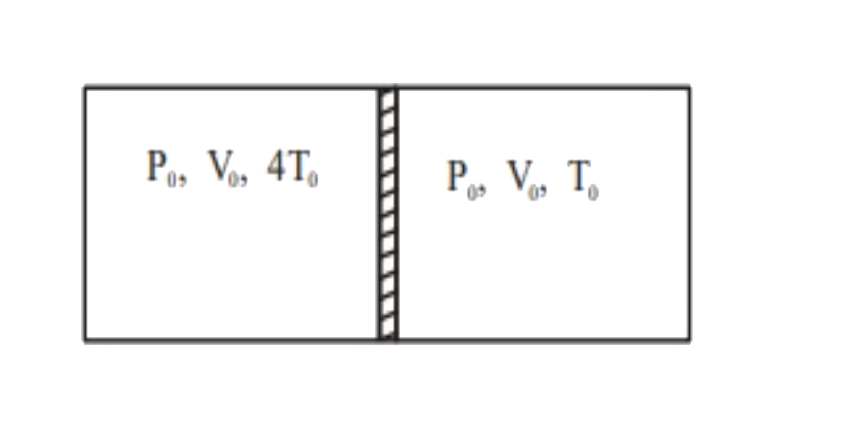A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A cylindrical adiabatic container of total volume 2V(0) divided into t...
Text Solution
|
- A thermally insulated chamber of volume 2V(0) is divided by a friction...
Text Solution
|
- A closed and isolated cylinder contains ideal gas. An adiabatic separa...
Text Solution
|
- A diathermic piston divides adiabatic cylinder of volume V0 into two e...
Text Solution
|
- Following figure shows on adiabatic cylindrical container of volume V(...
Text Solution
|
- Consider the adjacent figure. A piston divides a cylindrical container...
Text Solution
|
- A cylindrical container having nonconducting walls is partitioned in t...
Text Solution
|
- A cylindrical adiabatic container of total volume 2V(0) divided into t...
Text Solution
|
- A piston divides a closed gas cylinder into two parts. Initially the p...
Text Solution
|