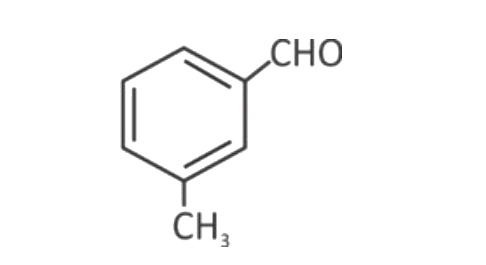
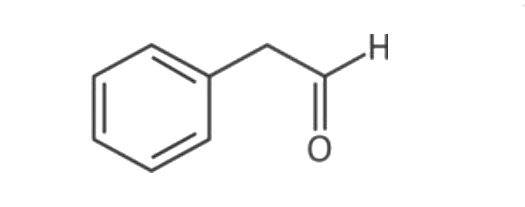
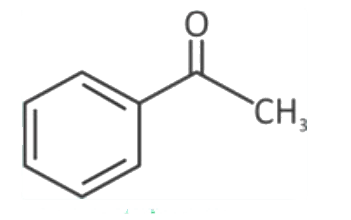
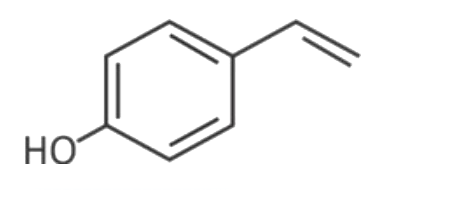
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A benzenoid organic compound A(C(8)H(8)O) gives B and white crystallin...
Text Solution
|
- An organic compound A upon reacting with NH(3) gives, B on heating, B ...
Text Solution
|
- An organic compound A’ with molecular formula C(7)H(7)NO reacts with ...
Text Solution
|
- An organic compound A, C(5)H(8)O reacts with H(2)O, NH(3) and CH(3)COO...
Text Solution
|
- Compound A is heated with alcoholic potash gives (CH(3))(2)C=CH-CH(3) ...
Text Solution
|
- An organic compound A upon reacting with NH(3) gives B. On heating B g...
Text Solution
|
- An organic compound (A) with molecular formula C(2)H(5)Cl reacts with ...
Text Solution
|
- A benzenoid organic compound A(C(8)H(8)O) gives B and white crystallin...
Text Solution
|
- An orgainc compound A upon reacting with NH(3) gives B On heating B gi...
Text Solution
|