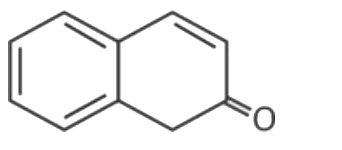
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Find the last product [D] in reaction sequence C(6)H(5)CHO+CH(3)-ove...
Text Solution
|
- The molecules CH(3)-overset(O)overset(||)C-CH(2) -overset(O)overset(||...
Text Solution
|
- CH(3)overset(O)overset(||)(C)CH(2) overset(CH(3))overset(|)underset(CH...
Text Solution
|
- In the reaction sequence CH(3)-overset(O)overset(||)(C)-H-underset(.^(...
Text Solution
|
- Which of the following are intermediates in the reaction of excess of ...
Text Solution
|
- Find the last product [D] in reaction sequence C(6)H(5)CHO+CH(3)-overs...
Text Solution
|
- For the given four structures , I to IV, which of the following is cor...
Text Solution
|
- Identify A,B and C in the following reactions CH(3)Cl overset(KCN) to ...
Text Solution
|
- Identify the major product in the following reactions : C(6)H(5)-CH(...
Text Solution
|