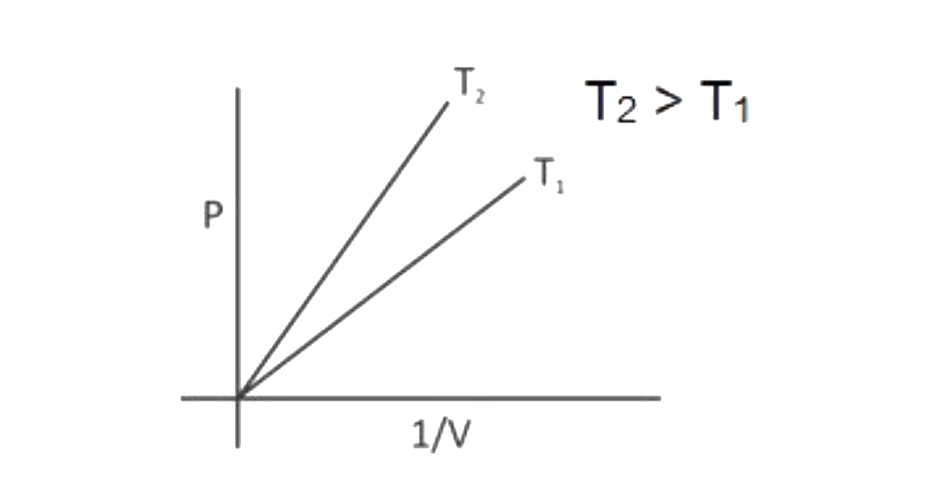
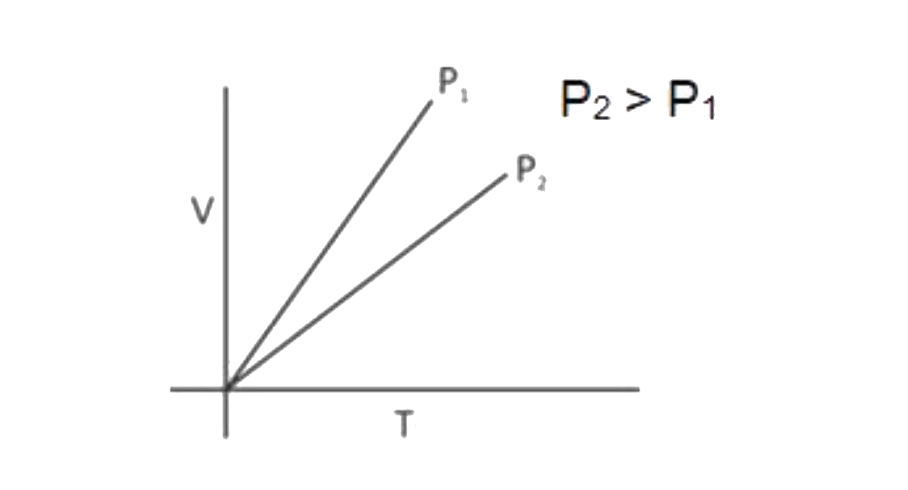
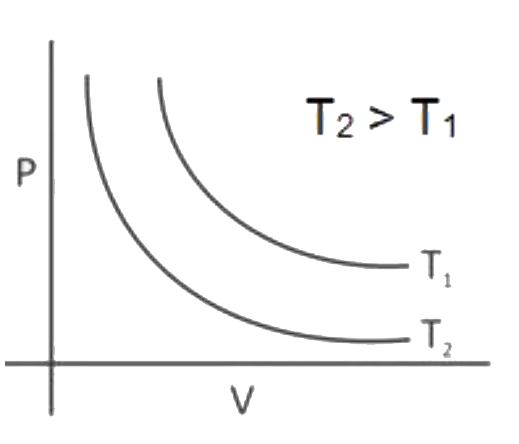
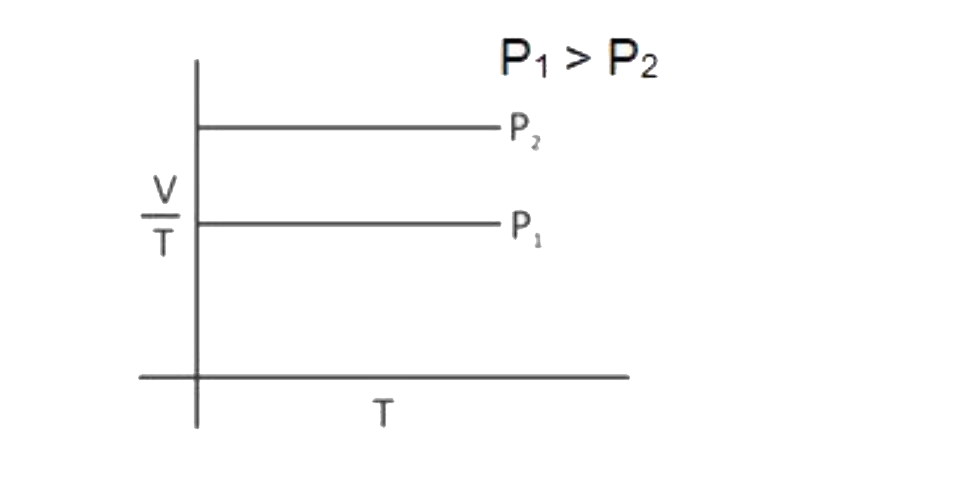
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following graphs is inconsistent with ideal gas behaviour...
Text Solution
|
- Which of the following graphs is consistent with ideal gas behaviour?
Text Solution
|
- Graph depicting correct behaviour of ideal gas and H(2) gas will be (n...
Text Solution
|
- Which of the following graphs is inconsistent with ideal gas behaviour...
Text Solution
|
- The graph that does not repersent the behaviour of an ideal gas is-
Text Solution
|
- The graph that does represent the behaviour of an ideal gas is :
Text Solution
|
- Which of the following graphs represent the behaviour of an ideal gas ...
Text Solution
|
- Which one of the following graphs correctly gives the ideal gas behavi...
Text Solution
|
- Which one of the following graphs represents the behaviour of an ideal...
Text Solution
|