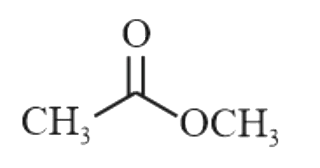
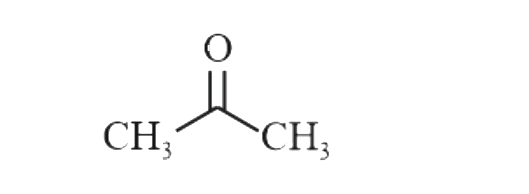
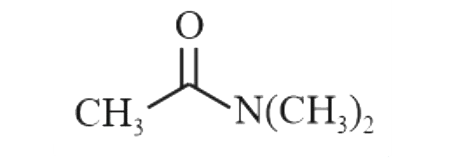
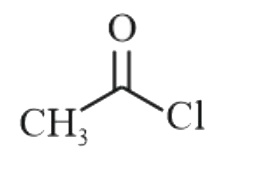
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The least electrolphilic sp^(2) carbon present in
Text Solution
|
- The number of sp^(2) hybridised carbons present in ''Aspartame'' is .
Text Solution
|
- Both sp^(2) " and " sp^(3) hybrid carbons are present in which of the ...
Text Solution
|
- How many sp^(2) and sp-hybridised carbon atoms are present respectivel...
Text Solution
|
- The least electrolphilic sp^(2) carbon present in
Text Solution
|
- The number of sp^(2) hybridized carbons present in "chloroxylenol" is
Text Solution
|
- The number of sp^(2) hybridized carbons present in "chloroxylenol" is
Text Solution
|
- how many sp^(2) carbon atoms are present in the final product?
Text Solution
|
- How many sp^(2) and sp-hybridised carbon atoms are present respectivel...
Text Solution
|