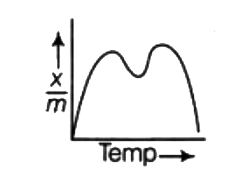
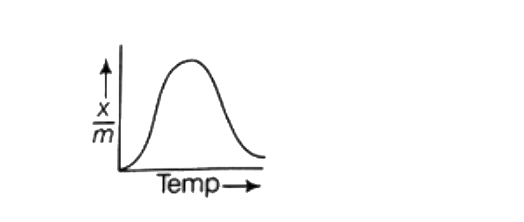
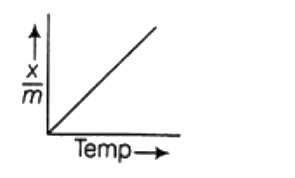
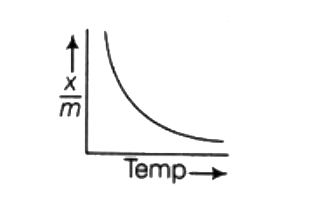
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following represents physical adsorption?
Text Solution
|
- Which of the following is an example of both physical adsorption and c...
Text Solution
|
- Which of the following represents physical adsorption?
Text Solution
|
- Which of the following represents correctly the variation of degree of...
Text Solution
|
- Which of the following is physical adsorption?
Text Solution
|
- Which of the following is a property of physical adsorption?
Text Solution
|
- Which of the following statements is not true for chemical adsorption ...
Text Solution
|
- Which equation represents Freundlich adsorption isotherm (physical ads...
Text Solution
|
- Which equation represents. Freundlisch adsorption isotherm (physical a...
Text Solution
|