A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- which of the following statement is correct for the reactivity in S(N)...
Text Solution
|
- Which of the following si most reactive toward S(N^(2)) reaction ?
Text Solution
|
- Which of the following is the correct order of decreasing S(N^2) react...
Text Solution
|
- Which of the following is the correct order of decreasing S(N^(2)) rea...
Text Solution
|
- Which of the following halides will be most reactive towards S(N^(2)) ...
Text Solution
|
- Which one of the following species will be most reactive in S(N^(2)) r...
Text Solution
|
- The correct increasing order of reactivity of halides for S(N)2 reacti...
Text Solution
|
- which of the following statement is correct for the reactivity in S(N)...
Text Solution
|
- Which of the following halides will be most reactive in S(N)1 reaction...
Text Solution
|
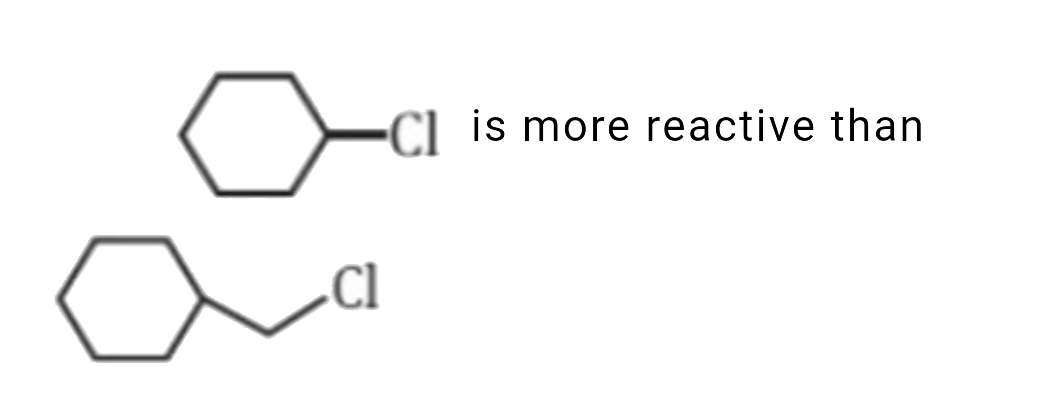
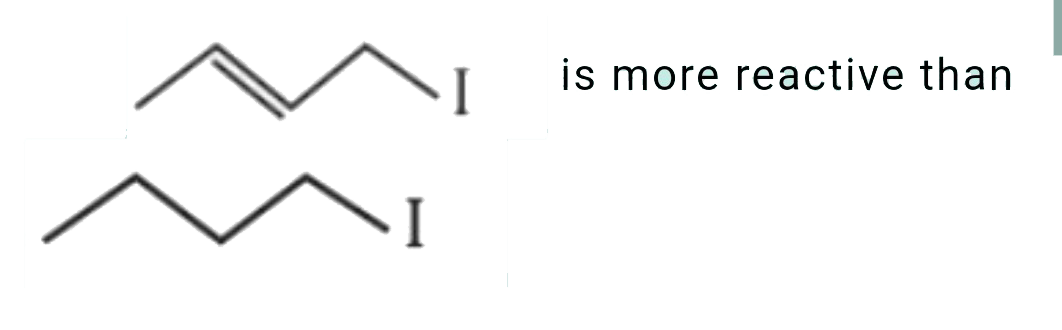
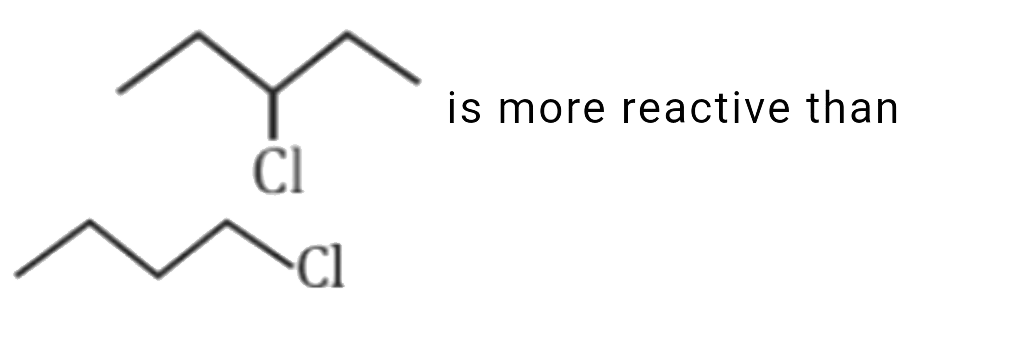
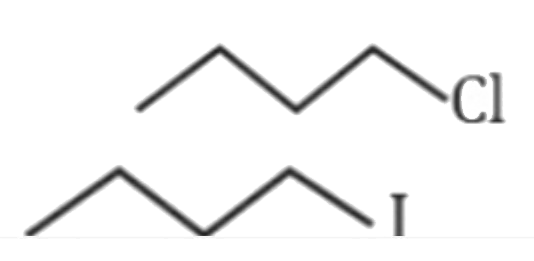 is more reactive than
is more reactive than