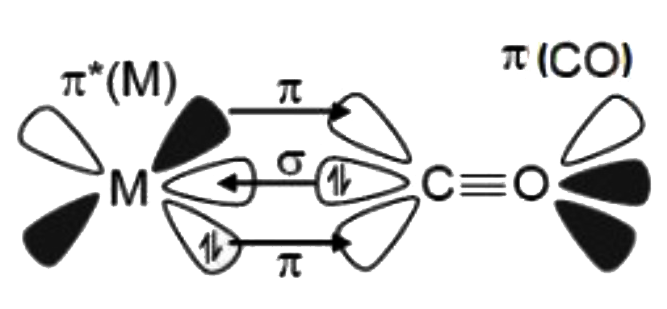
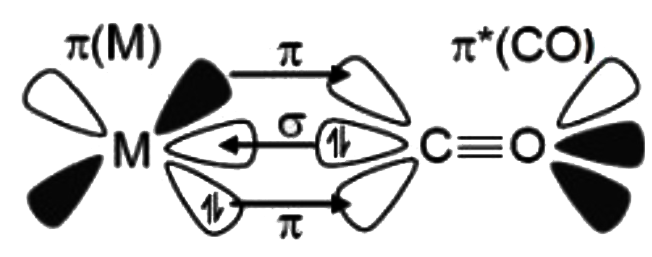
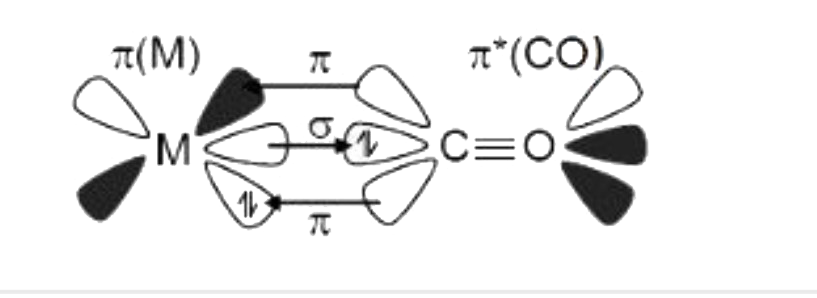
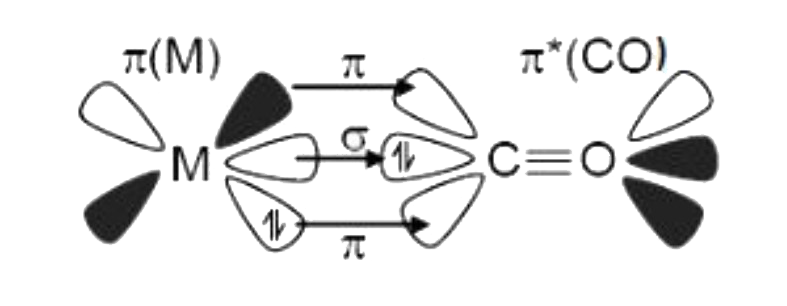
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Metal 'M' forms a carbonyl compound in which it is present in its lowe...
Text Solution
|
- Bonding In Metal Carbonyls
Text Solution
|
- Metal 'M' forms as carbonyl compound in which it is present in its low...
Text Solution
|
- In metal carbonyls, metal ligand sigma bond is formed by :
Text Solution
|
- In metal carbonyls, metal ligand sigma bond is formed by :
Text Solution
|
- Explain Bonding is metal carbonyls.
Text Solution
|
- In metal carbonyls, metal is present in oxidation state.
Text Solution
|
- Metal 'M' forms a carbonly compound in which it is present in its lowe...
Text Solution
|
- Which of the following is not a metal carbonyl?
Text Solution
|