A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
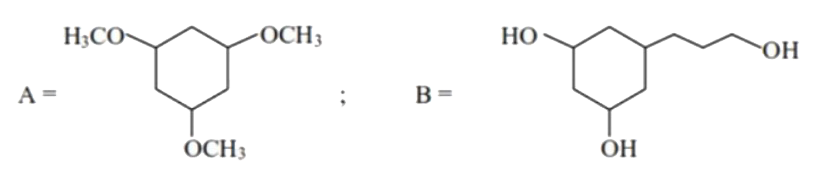
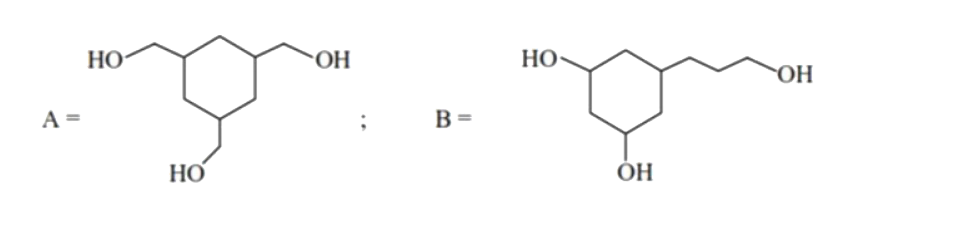
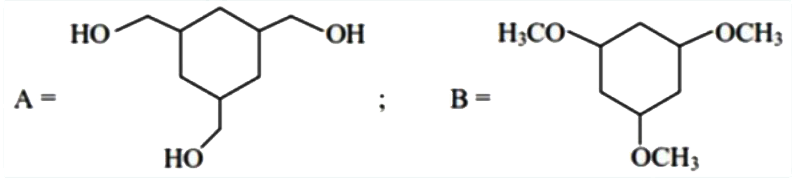
- There are two compounds A and B of molecular formula C(9)H(18)O(3). A ...
Text Solution
|
- An organic compound has molecular formula C(9)H(18). It is a saturated...
Text Solution
|
- An organic compound 'A' has the molecular formula C(3)H(6)O , it under...
Text Solution
|
- A compound A has the molecular formula C(5)H(9)Cl. It does not react w...
Text Solution
|
- An aromatic compound 'A' having molecular formula C(7)H(6)O(2) on trea...
Text Solution
|
- A compound (A) has molecular formula C(5)H(9)CI It does not react with...
Text Solution
|
- A compound (A) has molecular formula C(5)H(9)CI It does not rea...
Text Solution
|
- There are two compounds A and B of molecular formula C(9)H(18)O(3) . A...
Text Solution
|
- Molecular formula C(3)H(6)O(2) on acid hydrolysis gives A and B. The ...
Text Solution
|