A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
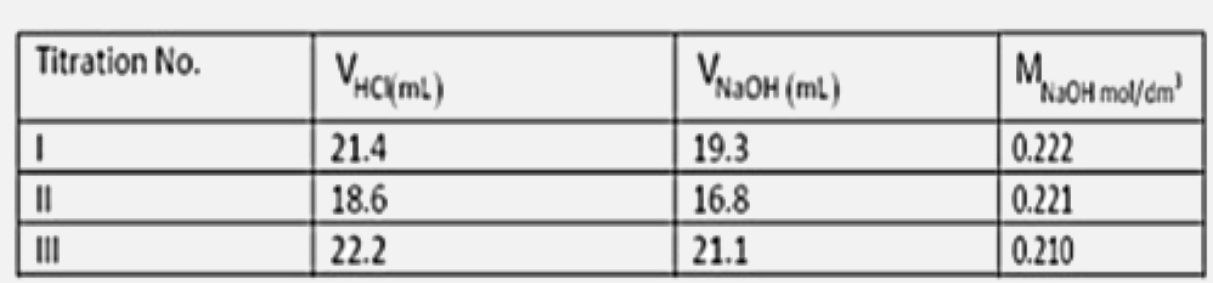
- The table below gives the results of three titrations carried out with...
Text Solution
|
- Using molarity as a conversion factor: An experiment calls for the add...
Text Solution
|
- Calculate the Quantity of substance in a Titrated solution: A flask co...
Text Solution
|
- If 100 mL of 1.00 M HCl and 100mL of 0.80 M NaOH solution are mixed, t...
Text Solution
|
- To determine molar solubility of an unknown metal hydroxide, M(OH)(3) ...
Text Solution
|
- To determine molar solubility of an unknown metal hydroxide, M(OH)(3) ...
Text Solution
|
- The table below shows the data for three titrations to determine the c...
Text Solution
|
- In the titration of NaOH and HCI, which of the following indicators wi...
Text Solution
|
- Assertion : In the titration of HCl against NaOH, phenolphthalein is u...
Text Solution
|