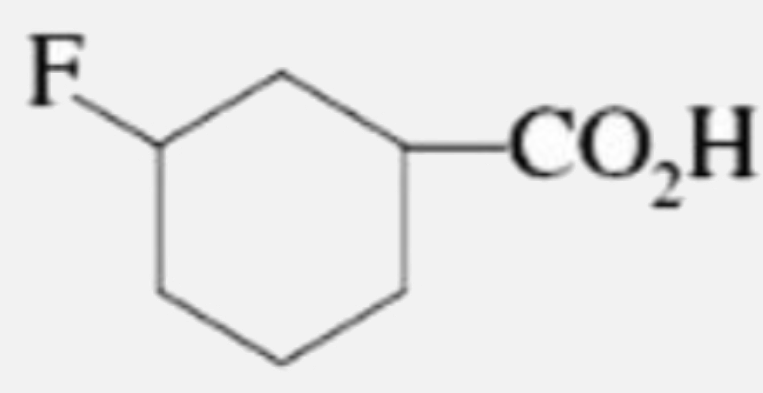
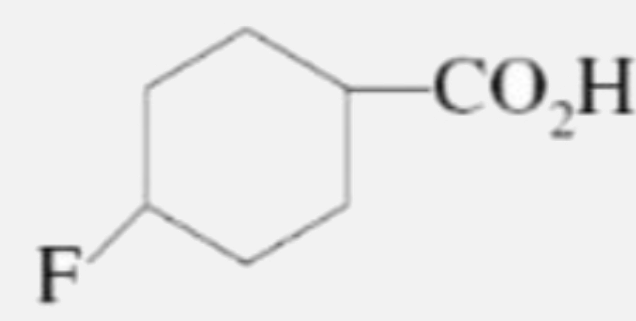
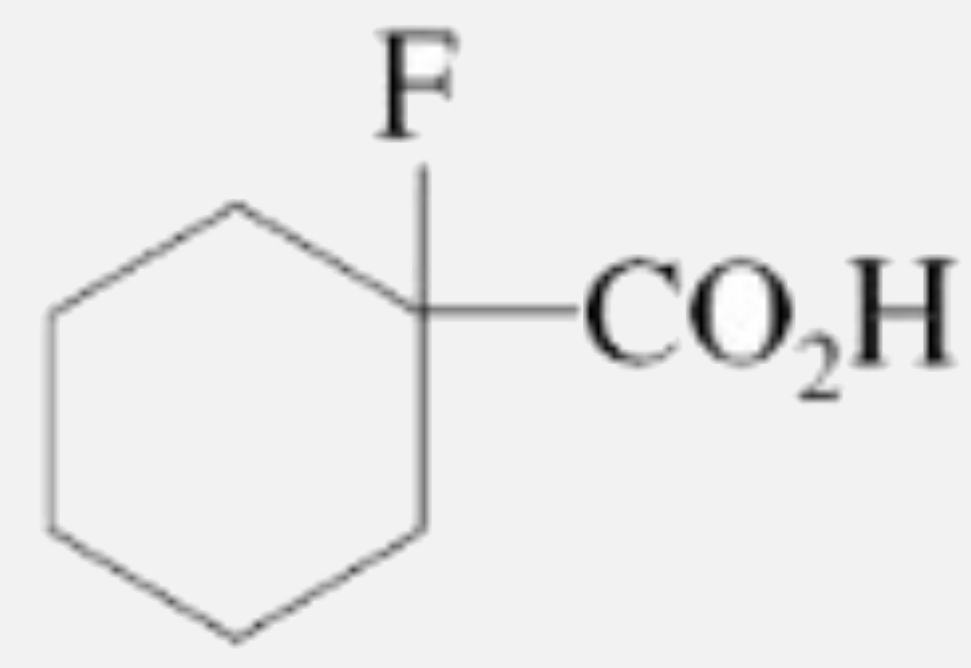
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following has highest K(a) value?
Text Solution
|
- Which of the following has the highest value of K(b) ?
Text Solution
|
- निम्नलिखित में किसका मान सबसे अधिक है ?
Text Solution
|
- Amongst the following hydroxides, the one which has the highest value ...
Text Solution
|
- Which of the following has the highest value of K(p)?
Text Solution
|
- Which of the following has highest K(a) value?
Text Solution
|
- Which of the following has highest value of K(sp) ?
Text Solution
|
- Which of the following has highest value of K(sp) ?
Text Solution
|
- निम्न में से किसकी रेडियोएक्टिवता का मान उच्चतम होता है
Text Solution
|