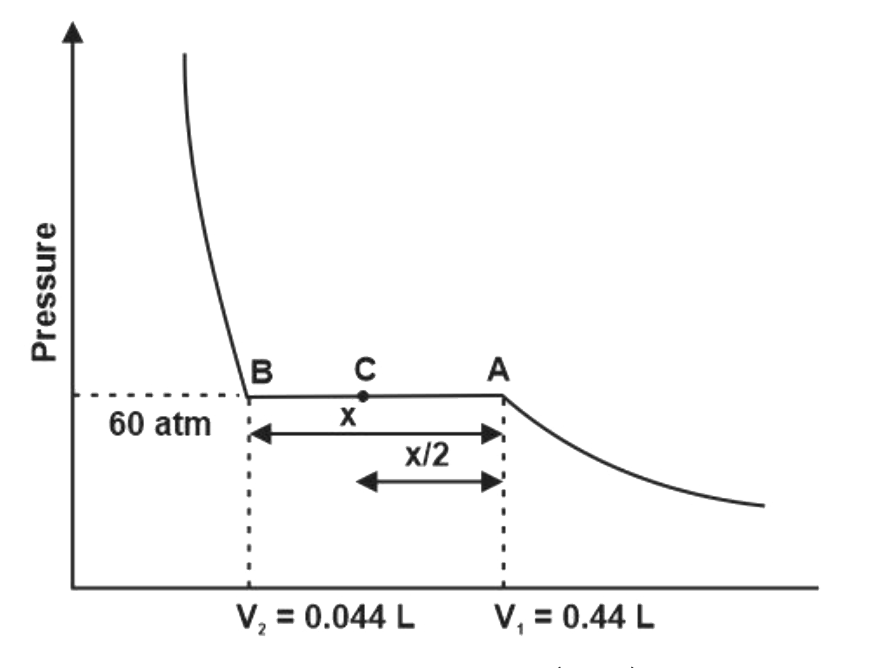
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- CO(2) gas is liquefaction for 1 mole of CO(2). Then which stateme...
Text Solution
|
- A gaseous mixture of H(2) and CO(2) gas contains 66 mass % of CO(2) . ...
Text Solution
|
- The density of CO(2) at 10 atm and 400K is 22g/L. the dominant force b...
Text Solution
|
- A bottle of cold drink has 200 mL liquid in which CO(2) is 0.1 molar. ...
Text Solution
|
- STATEMENT -1 : 18 ml of H(2)O and 18 ml of CO(2) at 277 K have same no...
Text Solution
|
- CO(2) के p-T प्रावस्था आरेख पर आधारित निम्नलिखित प्रश्नो के उत्तर दीजि...
Text Solution
|
- What is the density of CO(2) at 27 .^(@)C and 2.5 atm pressure ?
Text Solution
|
- A bottle of cold drink has 200 mL liquid in which CO(2) is 0.1 molar. ...
Text Solution
|
- 1 mole of CO(2) is available in the gaseous state (at NTP) 1 mole of C...
Text Solution
|