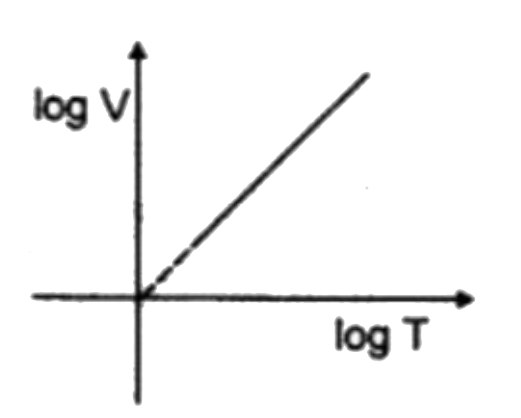
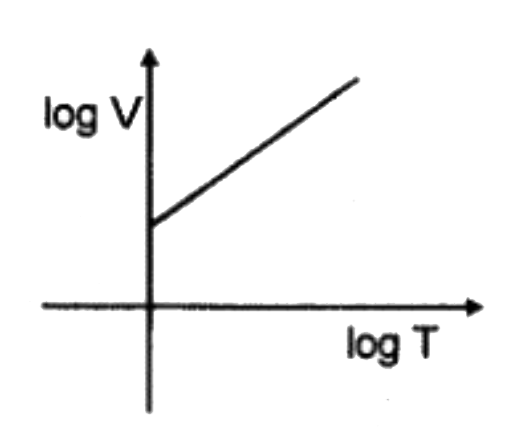
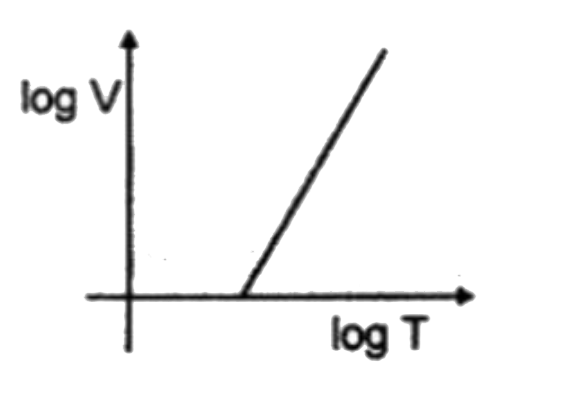
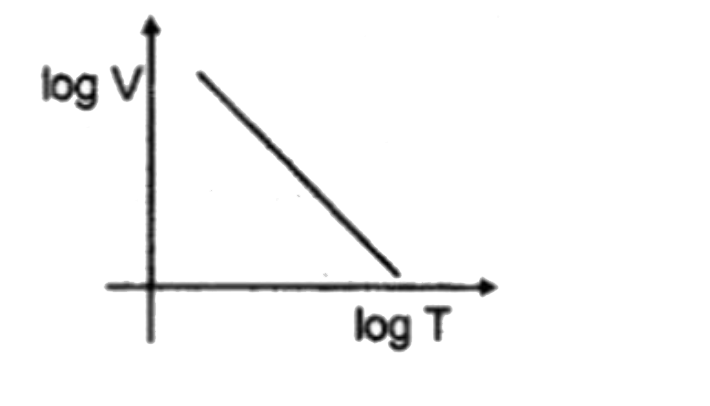
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following sketches is an isobars (Given : (nR)/(P) gt 1 )
Text Solution
|
- Which of the following sketches is an isobar ((nR)/Pgt1)
Text Solution
|
- Which among the following are isobars ?
Text Solution
|
- यदि P(A//B) gt P(A), तब निम्न में से कौन-सा सही है ?
Text Solution
|
- यदि P(A//B) gt P(A), तब निम्न में से कौन सही है ?
Text Solution
|
- Which of the following sketches is an isobars (Given : (nR)/(P) gt 1 )
Text Solution
|
- If P(A//B)gt P(A), then which of the following is correct :
Text Solution
|
- If P(A|B) gt P(A), then which of the following is correct:
Text Solution
|
- निम्न में से कौनसा समभारिक जोड़ा है
Text Solution
|