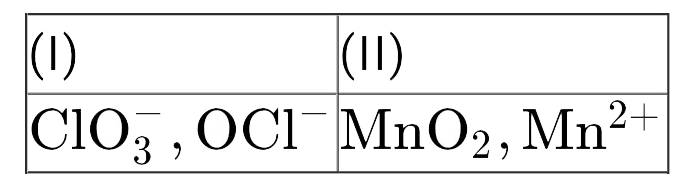
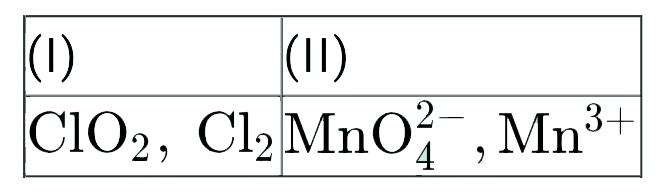A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Find out the compounds that will disproportionate in their aqueous sol...
Text Solution
|
- 4-Propene underset(778K)overset(Cl(2))rarr X overset(alc. NH(3))rarr Y...
Text Solution
|
- If overset(rarr)A=overset(rarr)B+overset(rarr)C and the magnitude of o...
Text Solution
|
- If overset(rarr)A=2 hat i + 3 hat j- hat k and overset(rarr)B=-hat i+3...
Text Solution
|
- A overset(Cl(2))(rarr) B overset("AgCN")(rarr) C overset([H])(rarr) (C...
Text Solution
|
- Find out the compounds that will disproportionate in their aqueous sol...
Text Solution
|
- Phenol overset("Raney nickel")rarr overset(CrO(3)//H(2)SO(4))rarr over...
Text Solution
|
- CH(4) underset(hv)overset(Cl(2))rarr A overset(Na)rarr B underset(hv)o...
Text Solution
|
- If |overset(rarr)A-overset(rarr)B|=|overset(rarr)A|-|overset(rarr)B| t...
Text Solution
|