A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- +underset("(Excess)")(CH(3)MgBr)rarr underset("(Gas)")(P)+Q How many...
Text Solution
|
- Ratio of C(p) and C(v) of a gas X is 1.4, the number of atom of the ga...
Text Solution
|
- Ratio of C(p) and C(v) of a gas 'X' is 1.4. The number of atoms of the...
Text Solution
|
- One gram mole of a gas at NTP occupies 22.4 litre as volume. This fact...
Text Solution
|
- At NTP, 10 litre of hydrogen sulphide gas reacted with 10 litre of sul...
Text Solution
|
- मोलर आयतन NTP पर किसी गैस (आदर्श ) के 1 मोल द्वारा घेरा गया आयतन है। ...
Text Solution
|
- The density of O2 at NTP is 1.429g / litre. Calculatethe standard mola...
Text Solution
|
- The volume occupied by 22.4 g of a gas ("vap. Density" = 11.2) at NTP ...
Text Solution
|
- At NTP, the mass of 1 litre of gas is 3 g. Molecular mass of the gas i...
Text Solution
|
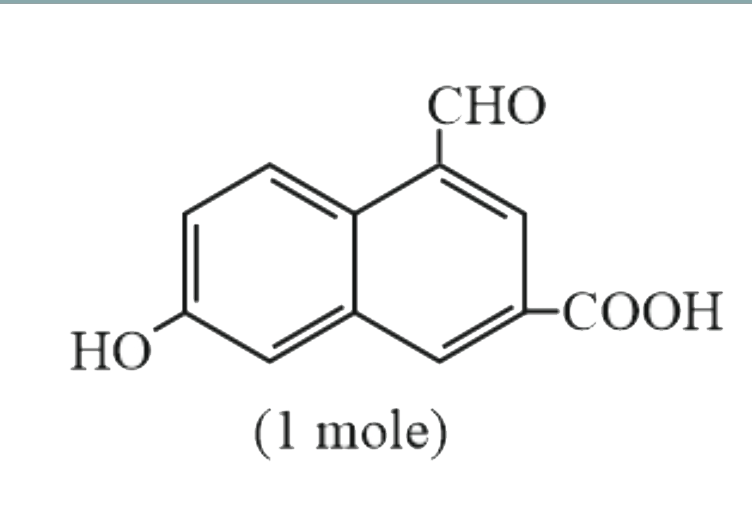 `+underset("(Excess)")(CH_(3)MgBr)rarr underset("(Gas)")(P)+Q`
`+underset("(Excess)")(CH_(3)MgBr)rarr underset("(Gas)")(P)+Q`