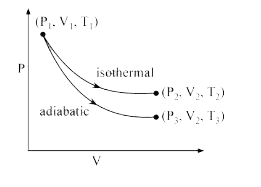
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The reversible expansion of an ideal gas under adiabatic and isotherma...
Text Solution
|
- The reversible expansion of an ideal gas under adiabatic and isotherma...
Text Solution
|
- Which of the following is correct option for the free expansion of an ...
Text Solution
|
- Which of the following is the correct option for the free expansion of...
Text Solution
|
- Which of the following is correct for free expansion of ideal gas unde...
Text Solution
|
- Which is the following is the correct option for free expansion of an ...
Text Solution
|
- Which expression is correct for the work done in adiabatic reversible ...
Text Solution
|
- Which is correct option for free expansion of an ideal gas under adiab...
Text Solution
|
- दिए हुए रेखाचित्र में एक आदर्श गैस के लिए रूद्धोष्म (adiabatic) और समत...
Text Solution
|