A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
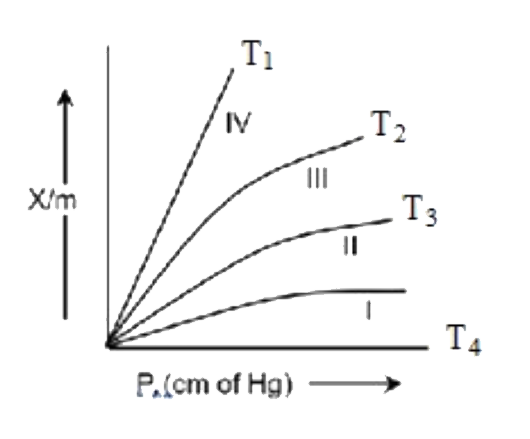
- The plots of the extent of adsroption (x/m) Vs pressure at different t...
Text Solution
|
- Give the decreasing order of the following in the extent of hydration ...
Text Solution
|
- The correct order of temperatures for a real gas is Boyle temp, Critic...
Text Solution
|
- The correct order of temperature for a real gas is {:("Boyle temp."...
Text Solution
|
- Velocity of sound in air (i) increases with temperature (ii) decreases...
Text Solution
|
- In the following sketch three constant pressure lines are plotted on T...
Text Solution
|
- Velocity of sound in air I. Increases with temperature II. Decreas...
Text Solution
|
- What type of curve are obatained when at constant temperature, we plot...
Text Solution
|
- A graph is plotted between extent of adsorption vs pressure (P) at con...
Text Solution
|