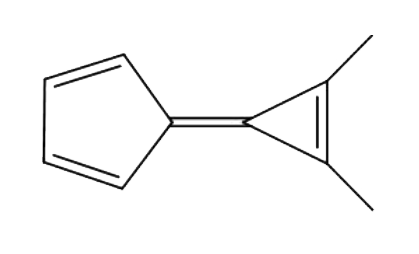
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Ordinarily the barrier to rotation about a carbon - carbon double bond...
Text Solution
|
- Which of the following will have the least hindered rotation about car...
Text Solution
|
- The restricted rotation about carbon-carbon double bond in 2-butene is...
Text Solution
|
- Which of the following has the lowest barrier to rotation about the in...
Text Solution
|
- The barrier for rotation about indicated bonds will be maximum in whic...
Text Solution
|
- Ordinarily the barrier to rotation about a carbon-carbon double bond i...
Text Solution
|
- Which of the following will have the least hindered rotation about car...
Text Solution
|
- Explain why rotation about carbon-carbon double bond is hindered ?
Text Solution
|
- The barrier for rotation about the indicated bonds Will be maximum i...
Text Solution
|