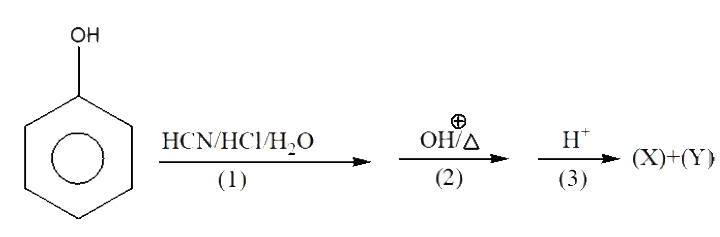A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- X gives white turbidity with Lucas reagent instantly. X and Y both tur...
Text Solution
|
- Which compound will not give white turbidity, immediately on reaction ...
Text Solution
|
- Alcohols which can give white turbidity immediately on reaction with L...
Text Solution
|
- Which of the following ions can turn red litmus solution blue ?
Text Solution
|
- What product is formed ions can turn red litmus solution blue ?
Text Solution
|
- Solution x turned blue litmus to red and Solution y turned red litmus ...
Text Solution
|
- Solution x turned blue litmus to red and Solution y turned red litmus ...
Text Solution
|
- Solution x turned blue litmus to red and Solution y turned red litmus ...
Text Solution
|
- Solution x turned blue litmus to red and Solution y turned red litmus ...
Text Solution
|