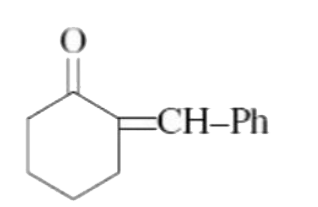
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- underset("Tollen's test")underset("Gives positive")underset(darr)(C(3)...
Text Solution
|
- underset((A))(MeCH)=O+underset((B))(H(2)NOH) overset(HCOONa)underset(D...
Text Solution
|
- underset(underset(underset(underset(underset("Fructose")(CH(2)OH))(|))...
Text Solution
|
- (i). Me-underset(OH)underset(|)(C)H-CH(2)-OH underset(Delta)overset(Hl...
Text Solution
|
- In the given reaction the products is : CH(3)-CH(2)-underset(O)underse...
Text Solution
|
- The reaction 2CH(3)-C-underset(O)underset(||)(C)C(2)H(5) overset(C(2...
Text Solution
|
- C(3)H(6)O underset(Delta) overset(OH^(-)) to Mesityl oxide . The C(3)H...
Text Solution
|
- underset(A)(C(3)H(6)Cl(2))overset("(i) KCN")underset(underset("(iii) "...
Text Solution
|
- Identify (A) in the following reaction sequence underset("iodoform tes...
Text Solution
|