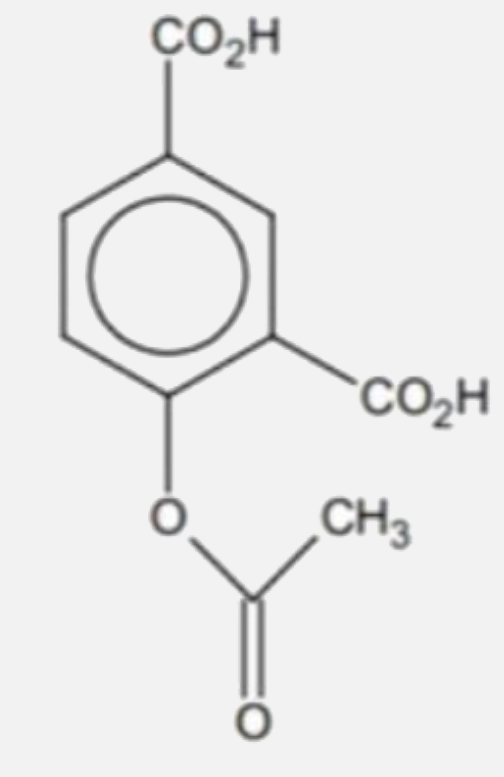
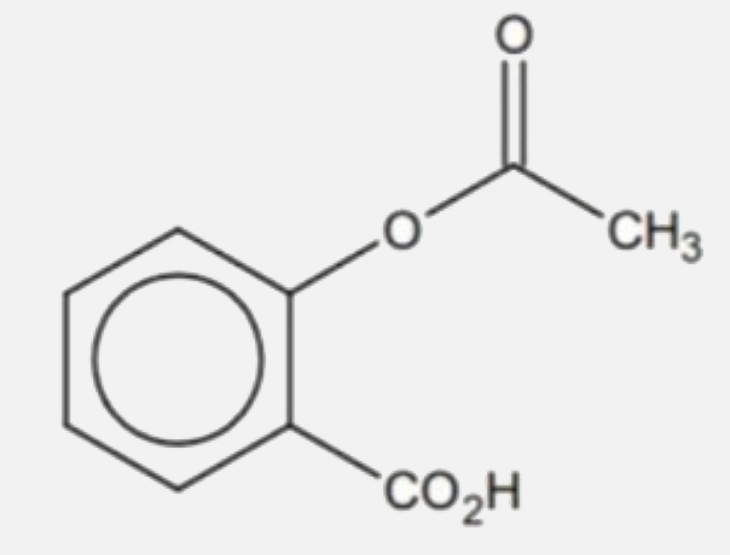
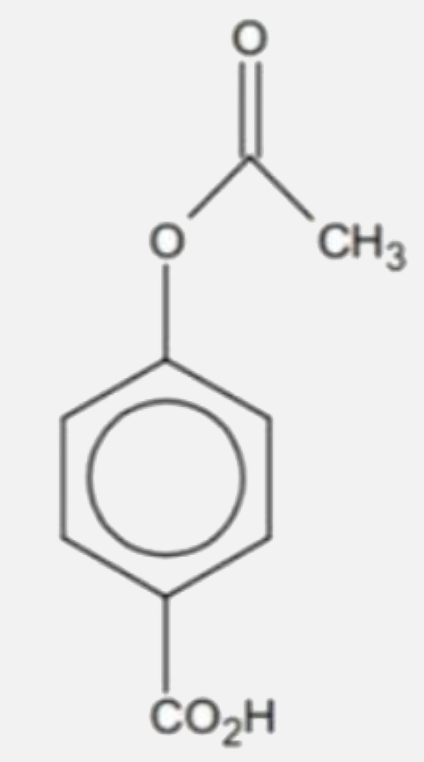
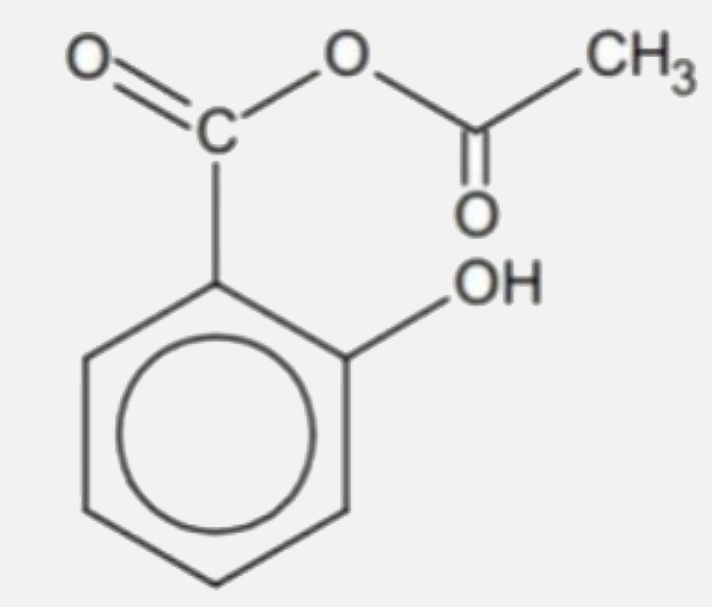
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Phenol on treatment with CO(2) in the presence of NaOH followed by aci...
Text Solution
|
- The reaction of compounf P with CH(3)MgBr (excess) in (C(2)H(5))(2)O f...
Text Solution
|
- Phenol on treatment with CO(2) in the presence of NaOH followed by aci...
Text Solution
|
- Calcium butanoate on heating followed by treatment with 1,2-ethandiol ...
Text Solution
|
- Benzene on reaction with conc. HNO(3) in presence of conc. H(2)SO(4) f...
Text Solution
|
- Oxidation of toluene with CrO(3) in the presence of (CH(3)CO)(2)O give...
Text Solution
|
- Treatment of benzene with CO/HCl in the presence of anhydrous "AlCl"(3...
Text Solution
|
- Treatment of benzene with CO/HCl in the presence of anhydrous "AlCl"(3...
Text Solution
|
- Oxidation of toluene with CrO(3) in the presence of (CH(3)CO)(2)O give...
Text Solution
|