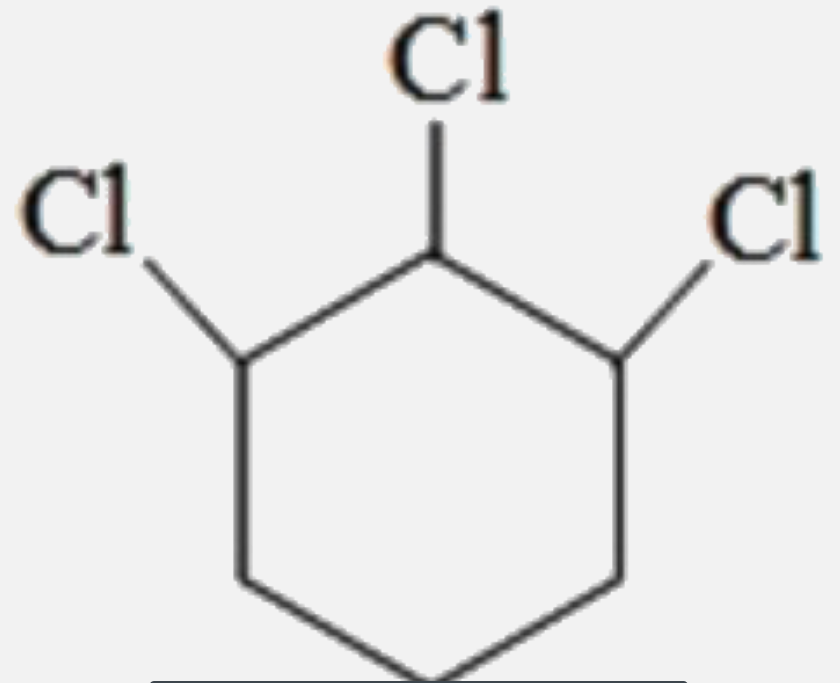
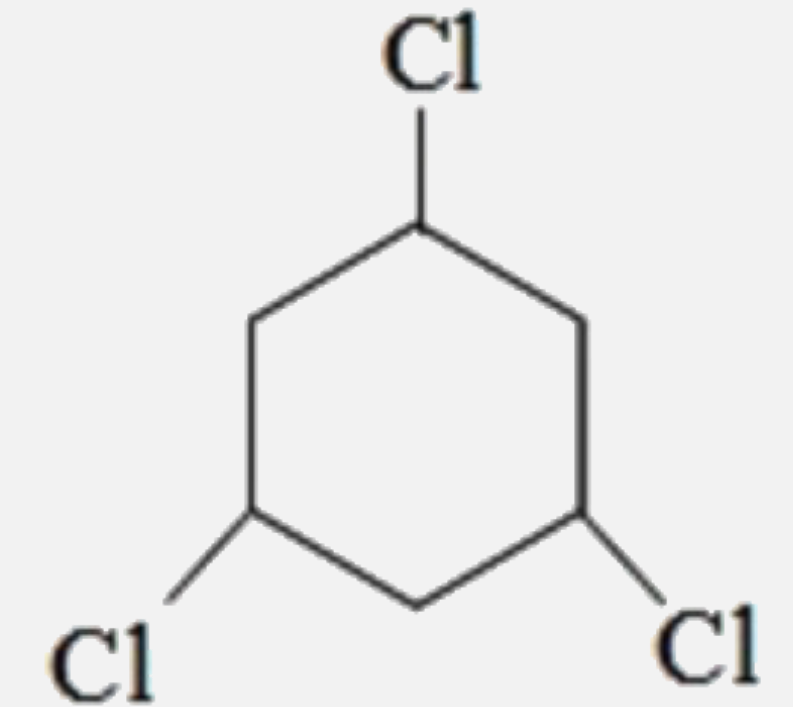
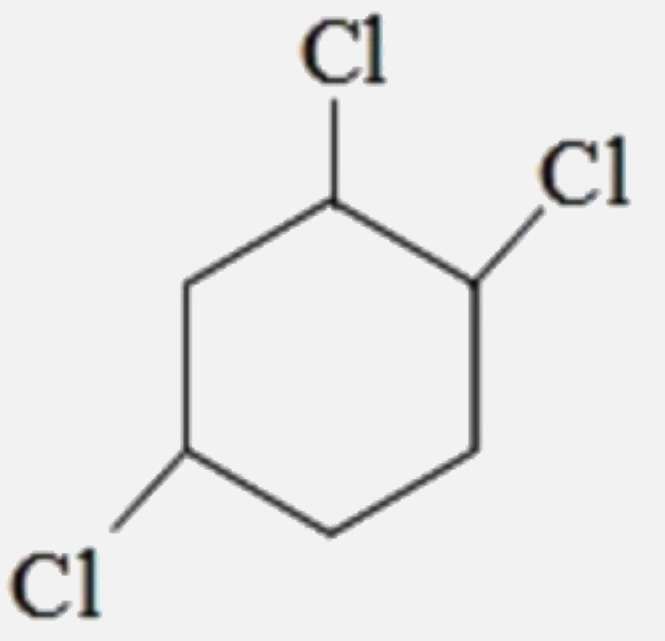
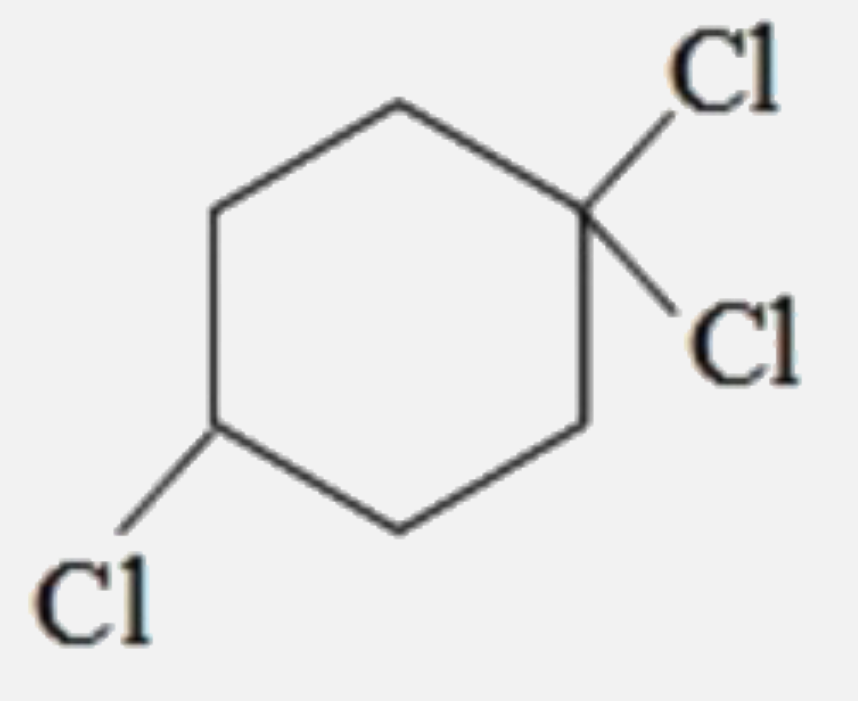
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following compounds does not have any geometrical isomer?
Text Solution
|
- Which of the following will have geometrical isomers ?
Text Solution
|
- Which of the following compounds exhibits geometrical isomers-
Text Solution
|
- Which of the following pairs of compounds are geometrical isomers
Text Solution
|
- Geometric isomers would be expected for which of the following compoun...
Text Solution
|
- No. of Geometrical isomers for following compound is :
Text Solution
|
- Which of the following does not exist as geometric isomers ?
Text Solution
|
- Which of the following compounds exhibit geometrical isomer ?
Text Solution
|
- निम्नलिखित में किस यौगिक के प्रकाशिक समावयव नहीं है ?
Text Solution
|