A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
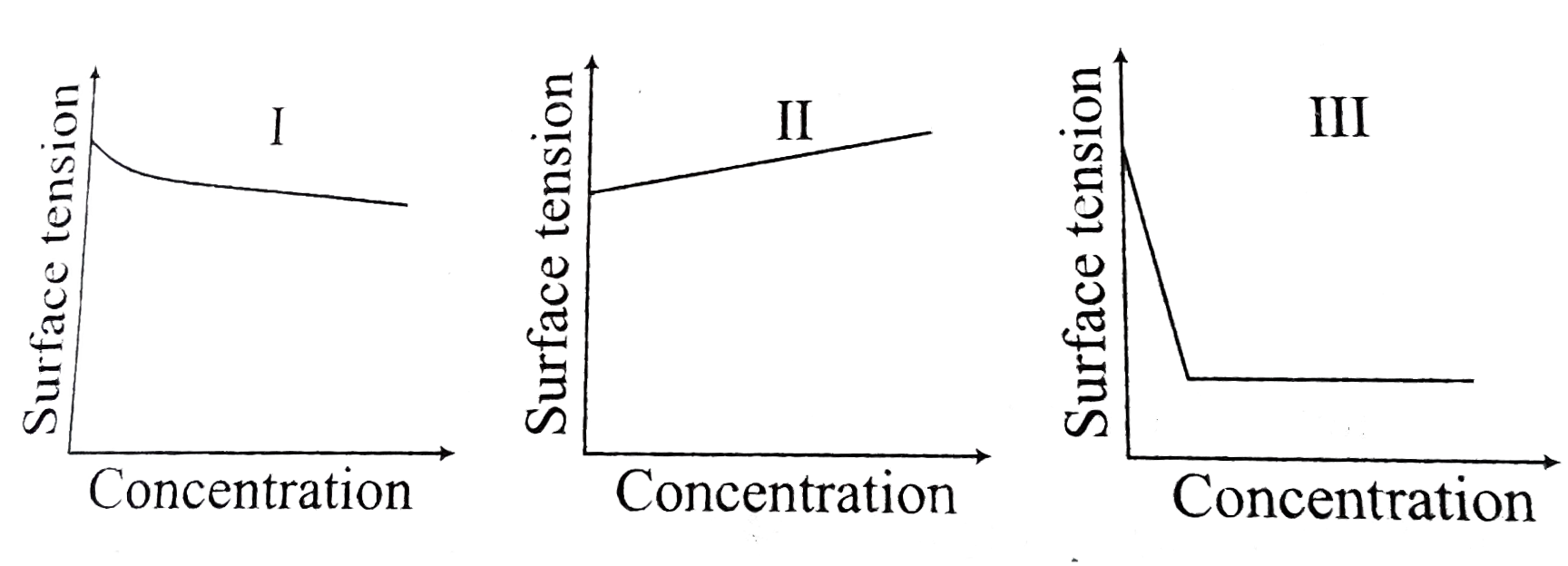
- The equalitative sketches I, II and III given below show the variation...
Text Solution
|
- Alcohols (i) CH(3)CH(2)CH(2)OH (ii) CH(3)-CHOH-CH(3) and (iii) CH(3)-C...
Text Solution
|
- The correct reactivity order of alcohols towards H-X will be (I) CH(2)...
Text Solution
|
- The equalitative sketches I, II and III given below show the variation...
Text Solution
|
- Compare the relative acidic strength of the following: (i) CH(3)OH (...
Text Solution
|
- Alcohols (i) CH(3)CH(2)CH(2)OH, (ii) CH(3)-CHOH-CH(3) and (iii) CH(3)-...
Text Solution
|
- CH(3)(CH(2))(10)CH(2)OSO(3)^(-)Na^(+) यौगिक में जलरागी एवं जलविरागि भा...
Text Solution
|
- Following compounds are given (i) CH(3)CH(2)OH (ii) CH(3)COCH(3) ...
Text Solution
|
- निम्नलिखित यौगिकों में जलरागि एवं जलविरागि भाग दर्शाइए | (a) CH(3)(C...
Text Solution
|