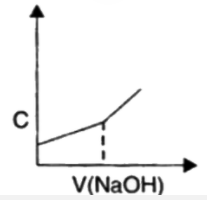
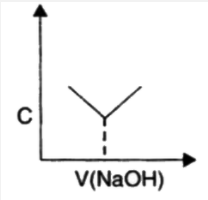
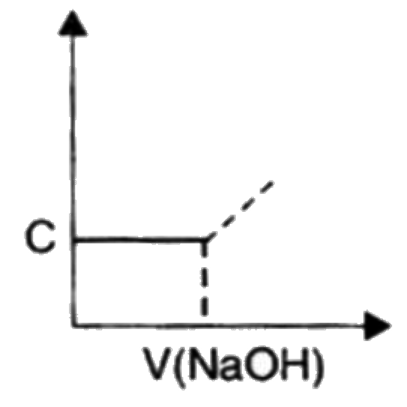
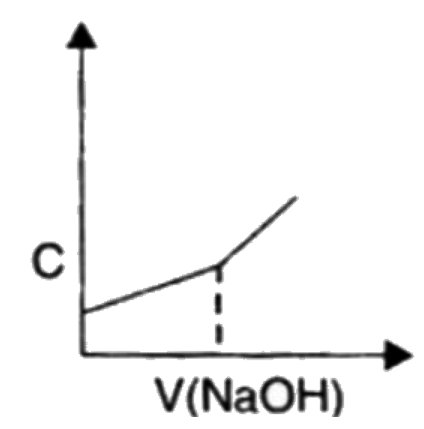
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following curve will represent the variation of conductan...
Text Solution
|
- 20 mol of M//10 CH(3)COOH solution is titrated with M//10 NaOH solutio...
Text Solution
|
- CH(3)COOH is titrated with NaOH solution. Which of the following state...
Text Solution
|
- 0.1M CH(3)COOH solution is titrated against 0.05M NaOH solution. Calcu...
Text Solution
|
- Calculate the pH at the equivalence point during the titration of 0.1M...
Text Solution
|
- Which one of the following curves represents the graph pH during the t...
Text Solution
|
- which one of the following curves represents the graph of pH dur...
Text Solution
|
- In the titration of CH(3)COOH against NaOH, we cannot use the :
Text Solution
|
- CH(3)COOH is neutralized by NaOH. Conductometric titration curve will ...
Text Solution
|