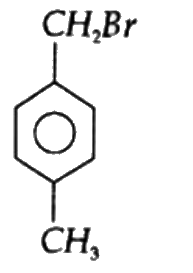
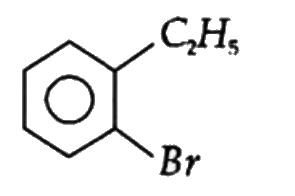
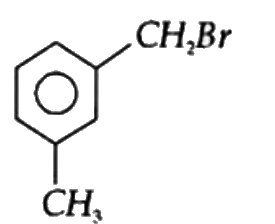
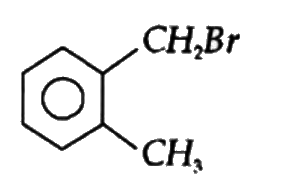
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Compound (A) C(8)H(9)Br. Gives a white precipitate when warmed with al...
Text Solution
|
- Compund (A)C(8)H(9) Br gives a white precipitate when warmed with alco...
Text Solution
|
- Compound (A) ,C(8)H(9)Br gives a white precipitate when warmed with a...
Text Solution
|
- Compound (A) C(8)H(9)Br , gives a light yellow precipitate when warmed...
Text Solution
|
- Compound (A) C(8)H(9)Br gives light yellow precipitate when warmed wit...
Text Solution
|
- Compound (A) ,C(8)H(9)Br gives a white precipitate when warmed with a...
Text Solution
|
- Compound (A), C(6)H(3)Br , gives a white precipitate when warmed with ...
Text Solution
|
- Compound (A) C(8)H(9)Br , gives a white precipitate when warmed with a...
Text Solution
|
- Compound (A) ,C(8)H(9)Br gives a white precipitate when warmed with a...
Text Solution
|