A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
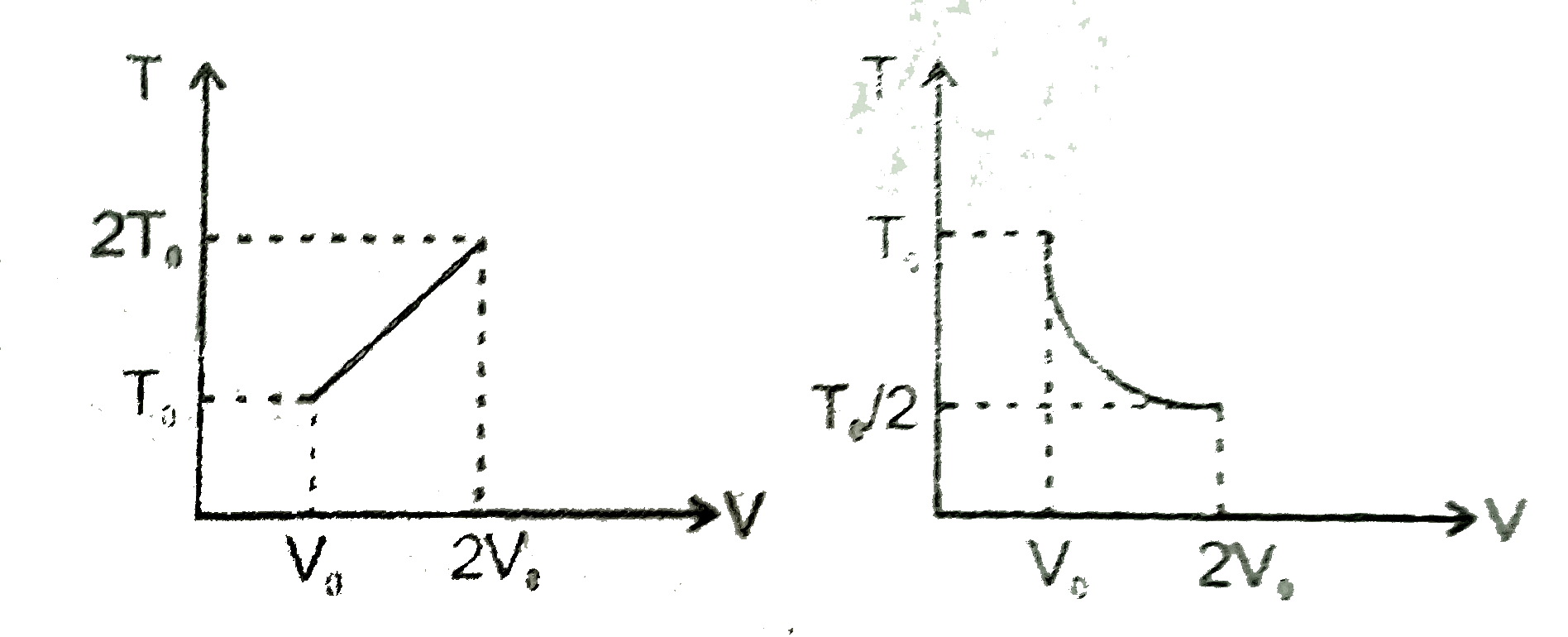
- For two thermodynamic process temperature and volume diagram are given...
Text Solution
|
- A gas is found to obey the law P^(2)V = constant. The initial temperat...
Text Solution
|
- For two thermodynamic process temperature and volume diagram are given...
Text Solution
|
- An ideal monatomic gas is at P(0), V(0). It is taken to final volume 2...
Text Solution
|
- Two moles of an ideal mono-atomic gas undergoes a thermodynamic proces...
Text Solution
|
- An ideal gaseous sample at intial state i(P(0),V(0),T(0)) is allowed t...
Text Solution
|
- A gas is found to obey the law P^(2) V= constant the innitial temperat...
Text Solution
|
- One mole of an ideal monoatomic gas undergoes the process T=T(0)+4V , ...
Text Solution
|
- One mole of a monatomic ideal gas undergoes a quasistatic process, whi...
Text Solution
|