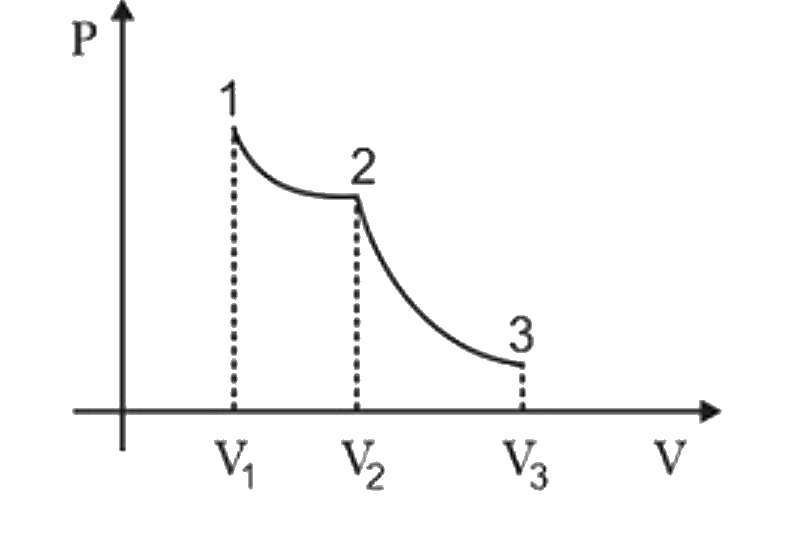
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- One mole of perfect gas, initially at a pressure and temperture of 10^...
Text Solution
|
- One mole of a perfect gas, initally at a pressure and temperature of 1...
Text Solution
|
- A certain volume of a gas (diatomic) expands isothermally at 20^(@)C u...
Text Solution
|
- A litre of hydrogen at 127^(@)C and 10^(6) dyne cm^(-2) pressure exp...
Text Solution
|
- A certain volume of a gas (diatomic) expandds isothermally at 20^(@)C ...
Text Solution
|
- A volume of gas at 15^(@)C expands adiabatically until its volume is d...
Text Solution
|
- A gass of given mass at a pressure of 10^(5)Nm^(-2) expands isothermal...
Text Solution
|
- One mole of a diatomic ideal gas at 300 K is heated at constant volume...
Text Solution
|
- An ideal gas at initial temperature 300 K is compressed adiabatically ...
Text Solution
|