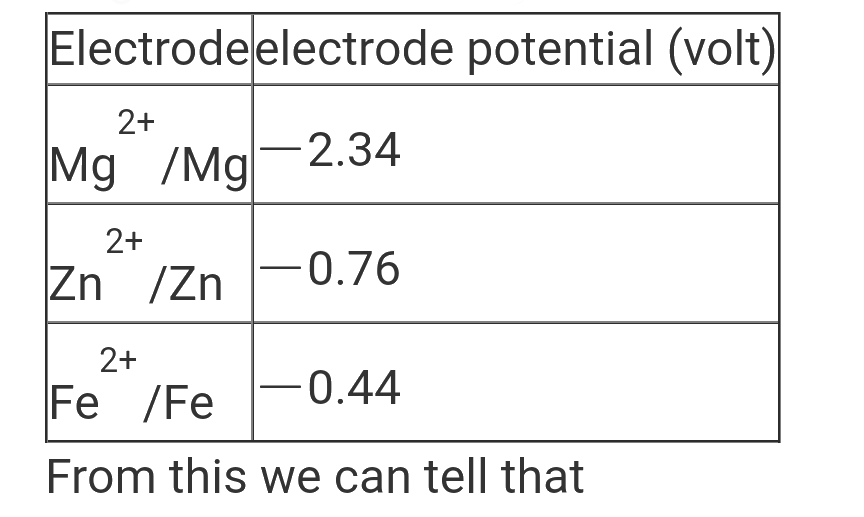
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The standard reduction potential at 298 K for single electrodes are gi...
Text Solution
|
- Standard electrode potential of SHE at 298 K is :
Text Solution
|
- The standard reduction potential of the Ag^(+)//Ag electrode at 298 K ...
Text Solution
|
- 298 K पर अर्ध्द – अभिक्रियाओं के मानक अपचयन विभव हैं-
Text Solution
|
- सिल्वर इलेक्ट्रोड के लिये मानक अपचयन विभव +0.80 वोल्ट है । गैल्वेनिक स...
Text Solution
|
- Standard reduction potential of the Ag^(+)|Ag electrode at 298 K is 0....
Text Solution
|
- The standard reduction potential of Zn-electrode is -0.76V. The standa...
Text Solution
|
- The standard reduction potential at 298 K for single electrodes are gi...
Text Solution
|
- Define standard electrode potential. If standard oxidation potential o...
Text Solution
|