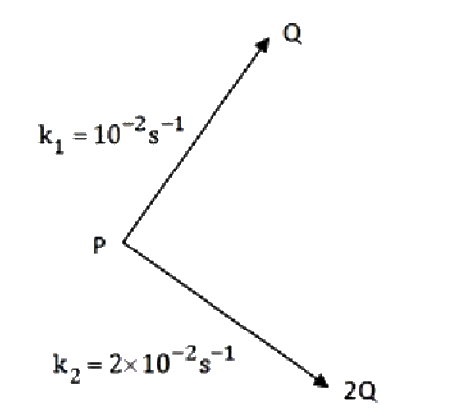
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Let the initial concentration of P is 1 M, the concentration of P afte...
Text Solution
|
- The rate constant for a zero order reaction is 2xx10^(-2) mol L^(-1) s...
Text Solution
|
- For the zero order reaction : A rarr P, K=10^(-2)("mol"//"litre")sec^(...
Text Solution
|
- For a zero order reaction : A rarr 2B, initial rate of a reaction is 1...
Text Solution
|
- Let the initial concentration of P is 1 M, the concentration of P afte...
Text Solution
|
- For the reaction p+qhArrr+s , initially concentrations of p and q are ...
Text Solution
|
- Reaction P rarr Q The half-life of the reaction and concentration of P...
Text Solution
|
- For the reaction, P((g))+3Q((g))iff4R((g)) Initial concentration of ...
Text Solution
|
- The rate constant for a zero order reaction is 2xx10^(2) mol L^(-1) "s...
Text Solution
|