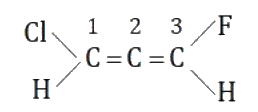A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the following molecule If pi- electron cloud of C(1)-C(2...
Text Solution
|
- Assertion : The double bond in C(2) molecule consider of both pi bonds...
Text Solution
|
- The pi electron cloud of C(1)-C(2) is present in the plane of paper th...
Text Solution
|
- Which of the following molecule is called buckninsterullerence? C(90),...
Text Solution
|
- Consider the following statements : 1. The distance between the line...
Text Solution
|
- निम्नलिखित कथनों पर विचार कीजिए I. .^(10)C(0)+(.^(10)C(0)+.^(10)...
Text Solution
|
- The electronic configuration of carbon is 1s^(2) 2s^(2) 2p^(2) . There...
Text Solution
|
- निम्नलिखित कथनों पर विचार कीजिए 1 . रेखाओं y = mx + c(1) और y = mx +...
Text Solution
|
- What is the total number of sigma and pi bonds in the following molecu...
Text Solution
|