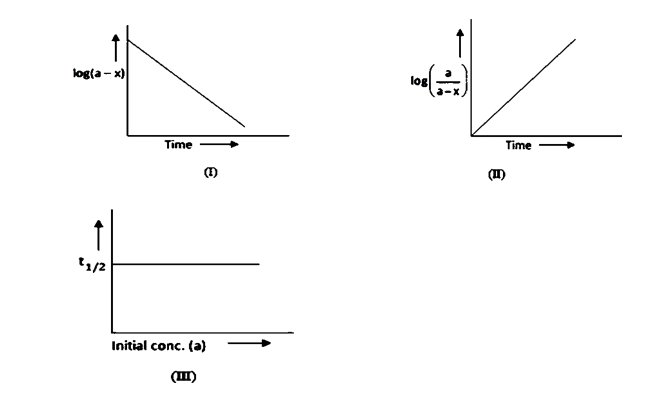
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following is/are correct for the first order reaction ? (...
Text Solution
|
- The half-life of a first order reaction is 30 min and the initial conc...
Text Solution
|
- The half life of a first order reaction is 30 min and the initia...
Text Solution
|
- Which of the followin ng fraphs represent a first order reaction (a= i...
Text Solution
|
- The initial concentration of the reactant in a first order reaction is...
Text Solution
|
- How does the initial concentration of the reactant in a first order re...
Text Solution
|
- Show that the time of a first order reaction takes for 'the nth' fract...
Text Solution
|
- Plot a graph of concentration of a reactant at time t, [ (a-x)] agains...
Text Solution
|
- For a first order reaction, the concentration of reactant :
Text Solution
|