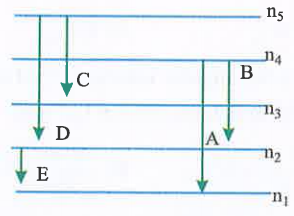
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For a hypothetical H like atom which follows Bohr's model, some spectr...
Text Solution
|
- In the emission spectrum of H- atom from energy level 'n' to ground s...
Text Solution
|
- For a H-like atom which Bohr's model some spectral lines were observed...
Text Solution
|
- The number of lines of Balmer series of H-atom that belong to visible ...
Text Solution
|
- For a hypothetical H like atom which follows Bohr's model, some spectr...
Text Solution
|
- For a hypothetical H like atom which follows Bohr's model, some spectr...
Text Solution
|
- The longest wavelength transitoin in one of the spectral series of hyd...
Text Solution
|
- Which one of the following series of line is found in the UV region of...
Text Solution
|
- Whwn the electron in an excited hydrogen atom jumps from an energy lev...
Text Solution
|