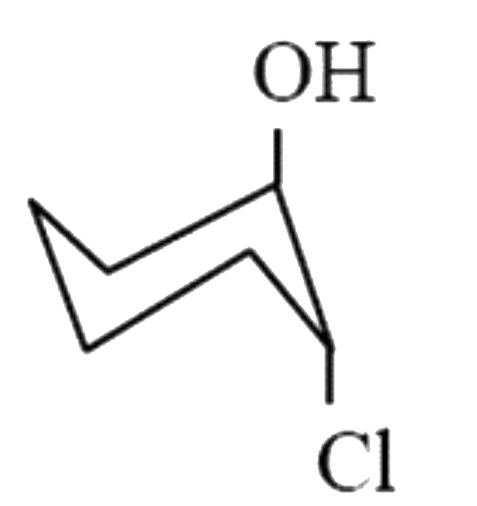
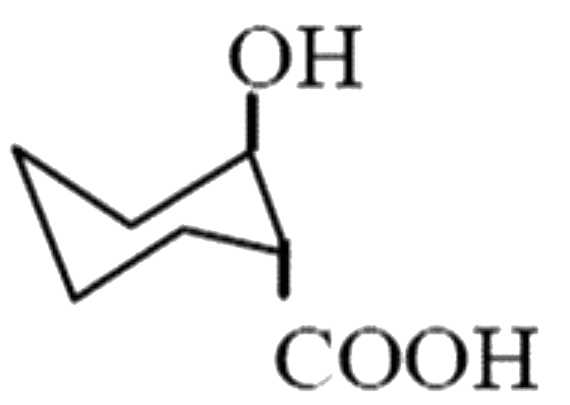
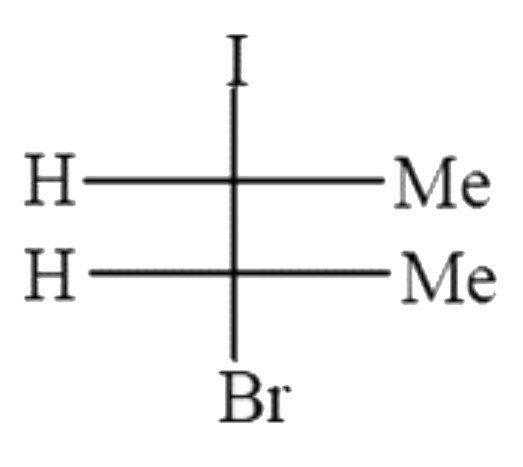
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- NGP (neighouring group of participation) assistance for S(N)2 reaction...
Text Solution
|
- S(N^(1))and S(N^(2)) reactions are
Text Solution
|
- S(N^(2)) reaction are
Text Solution
|
- Alkyl halides can participate in both S(N^(1)) and S(N^(2)) reactions ...
Text Solution
|
- Alkyl halides can participate in both S(N^(1)) and S(N^(2)) reactions ...
Text Solution
|
- The correct decreasing order of reactivity for a given (R) group in b...
Text Solution
|
- S(N^(1)) अभिक्रियाओं में कौन-सी क्रियाकारी स्पीशीज भाग लेती हैं?
Text Solution
|
- Correct order of leaving group ability for S(N)2 reaction is :
Text Solution
|
- tert - Butyl chloride (Me(3)C CI) does not participate in S(N)^(2) rea...
Text Solution
|