Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
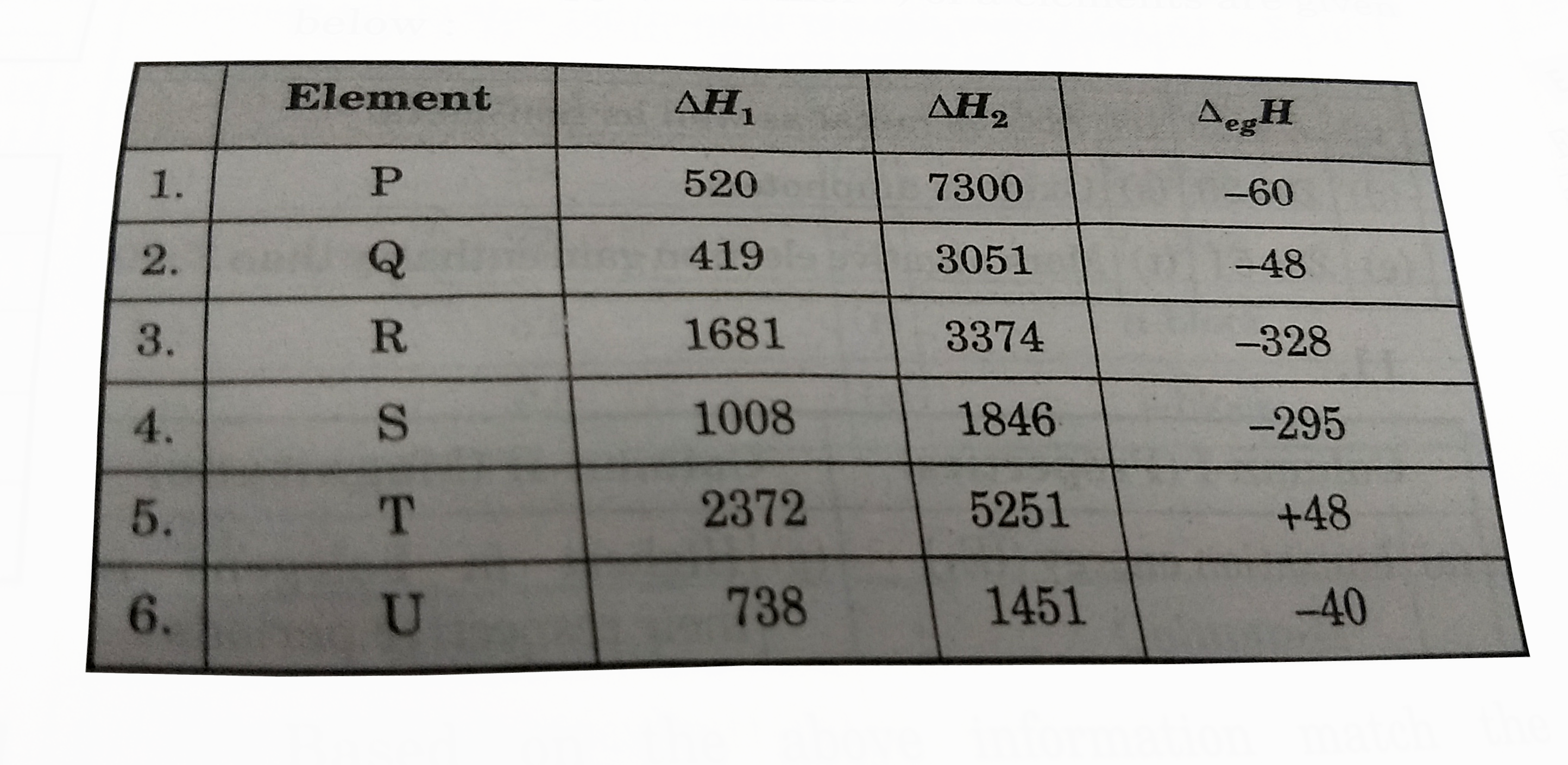
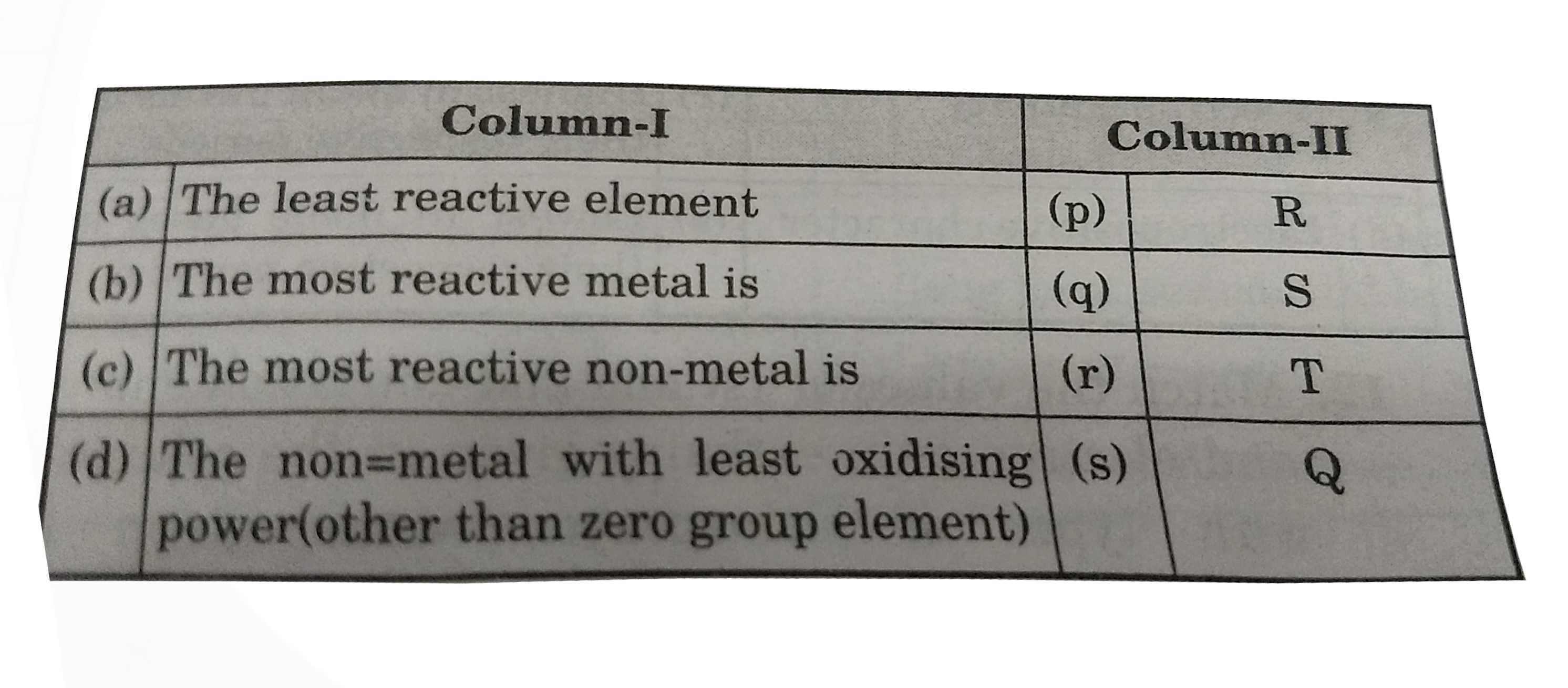
- The first (DeltaH(1)) and second (DeltaH(2)) ionisation enthalpies (in...
Text Solution
|
- Find bond enthalpy of C=O (in kJ/mol) using following information : ...
Text Solution
|
- Given C(2)H(2)(g)+H(2)(g)rarrC(2)H(4)(g): DeltaH^(@)=-175 " kJ mol"^(-...
Text Solution
|
- The first (DeltaH(1)) and second (DeltaH(2)) ionisation enthalpies (in...
Text Solution
|
- Calculate lattice energy for the change, Li^(+)(g)+Cl^(-) (g) rarr L...
Text Solution
|
- The first (DeltaIH1) and second (DeltaIH2) ionisation enthalpies (in k...
Text Solution
|
- Consider the following Born-Haber's cycle: (Where DeltaH(1),DeltaH(2),...
Text Solution
|
- Which ions are present in MgO(s) Calculate the enthalpy change for the...
Text Solution
|
- Construct a Born-Haber cycle and use it to calculate the first electro...
Text Solution
|