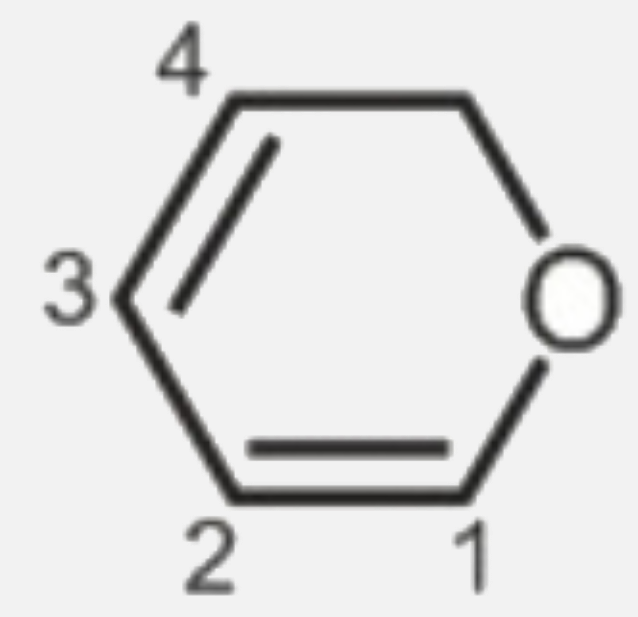
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In this molecules, pi- electron - density is more on
Text Solution
|
- In which of the following molecules pi -electron density in ring is ma...
Text Solution
|
- In which of the following molecules pi -electron density in ring is mi...
Text Solution
|
- Which molecules I showing pi -electrons alternate to pi -electron conj...
Text Solution
|
- In which of the following molecules pi-electron density in ring is min...
Text Solution
|
- In which of the following molecules pi -electron density in ring is ma...
Text Solution
|
- The number of pi-"electrons" in benzene molecule is :
Text Solution
|
- In this molecules, pi -electron density is more on :
Text Solution
|
- In which of the following molecules pi -electron density in ring is mi...
Text Solution
|