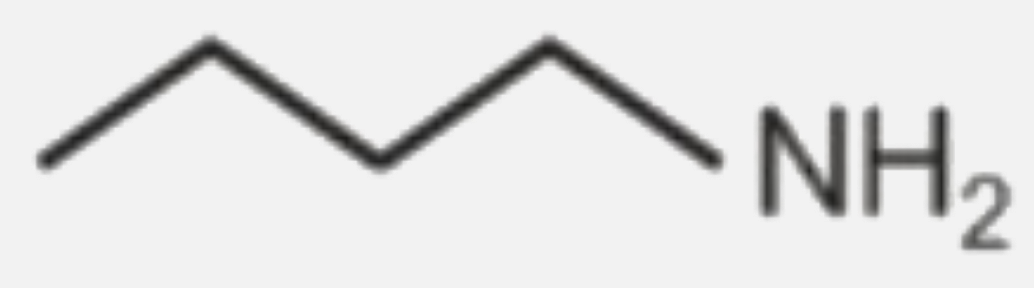
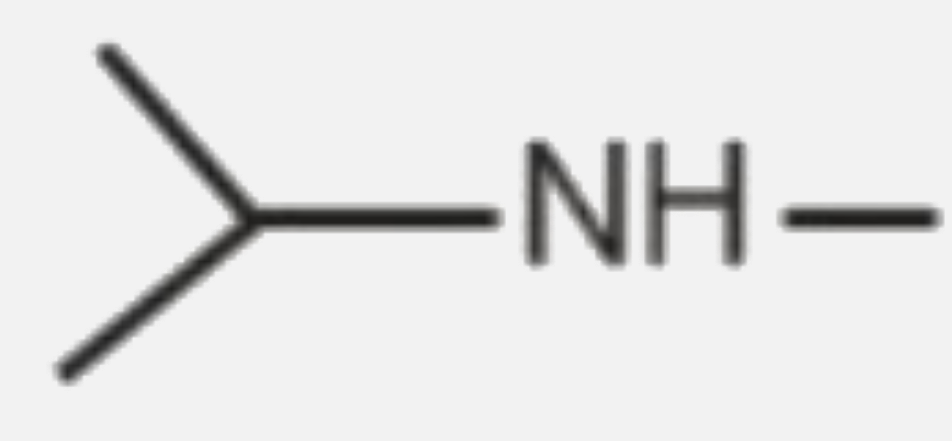
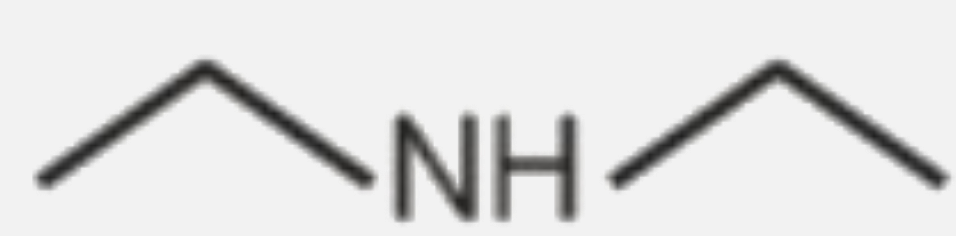
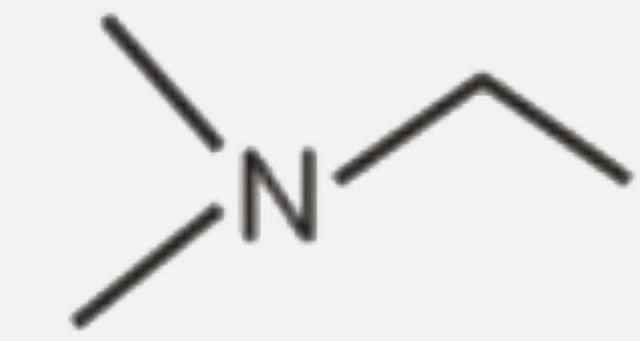
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Among the following isomeric amines which one is expected to have the ...
Text Solution
|
- Among the following amines namely ethylmethyl amine, propyl amine trim...
Text Solution
|
- Among the following isomeric amines which one is expected to have the ...
Text Solution
|
- Among the isomeric amines select the one with the lowest boiling point...
Text Solution
|
- Which (isomeric ) amine has lowest boiling point?
Text Solution
|
- Among the following select the alkane that is expected to have lowest ...
Text Solution
|
- Among the following select the alkane that is expected to have lowest ...
Text Solution
|
- Which one of the following isomeric amines has the highest boiling poi...
Text Solution
|
- Which one of the following isomeric amines has the highest boiling poi...
Text Solution
|