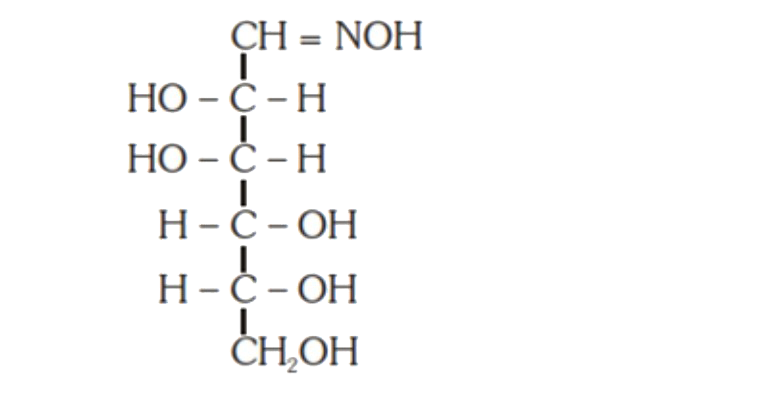
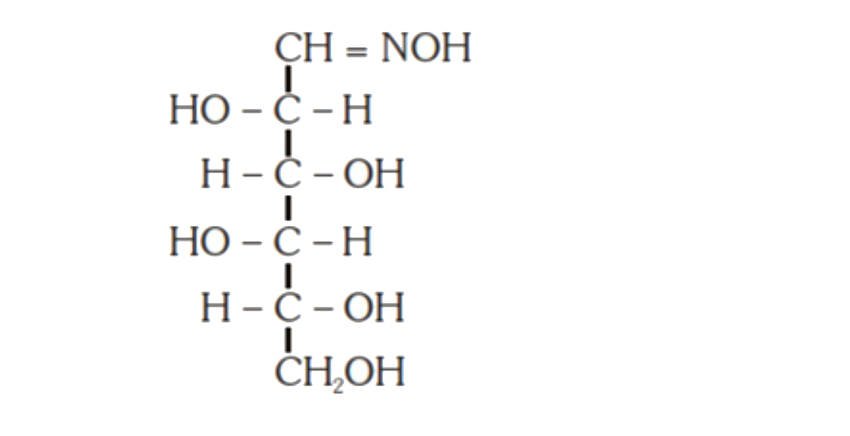
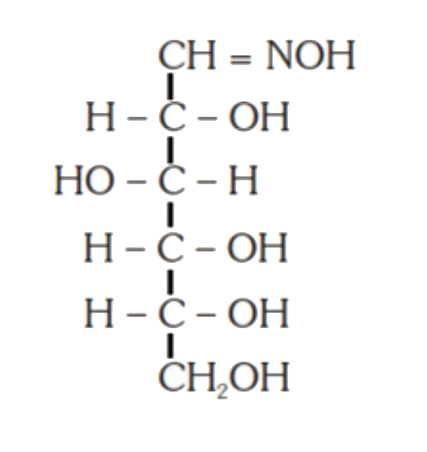
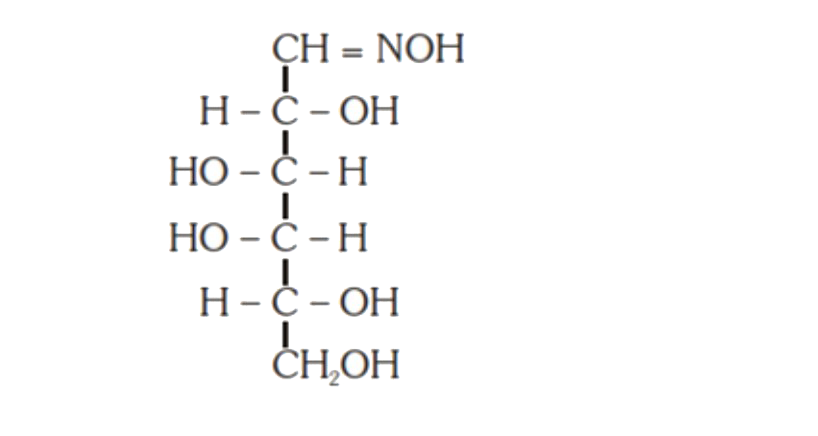A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- D-(+)-glucose reacts with hydroxyl amine and yields an oxime. The stru...
Text Solution
|
- Acetophenone on reaction with hydroxyl amine hydrochloride can produce...
Text Solution
|
- D(+) glucose reacts with hydroxylamine and yields an oxime. The struc...
Text Solution
|
- Assertion: Oximes are less acidic than hydroxyl amine. Reason: Oxim...
Text Solution
|
- D(+) glucose reacts with hydroxylamine and yields an oxime. The struc...
Text Solution
|
- ऑक्सीम, हाइड्रॉक्सिल ऐमीन से अधिक अम्लीय होती है, क्यो?
Text Solution
|
- D-(+)- glucose reacts with hydroxylamine and yields an oxime. The stru...
Text Solution
|
- Which one of the following gives oxime with hydroxyl amine?
Text Solution
|
- D(+) glucose reacts with hydroxylamine and yields an oxime. The struc...
Text Solution
|