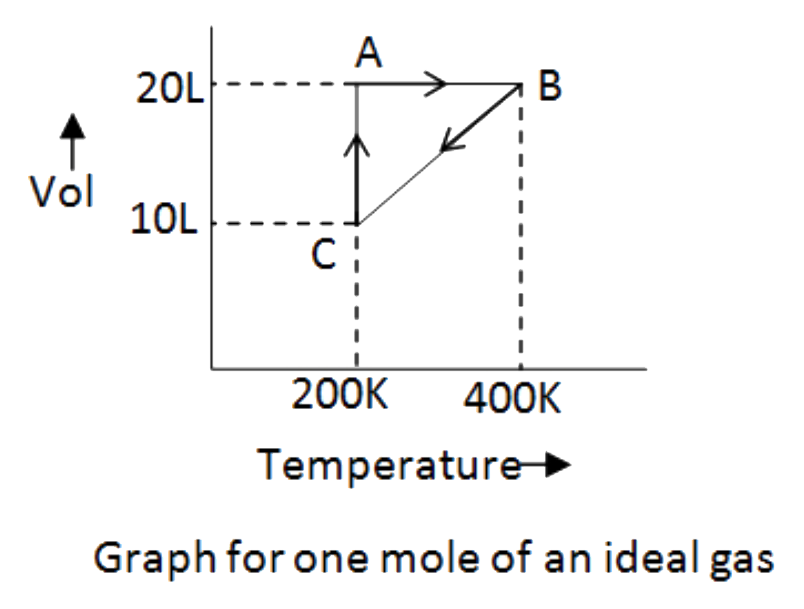
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Find work done in the irreversible process C to A.
Text Solution
|
- Work Done In Irreversible Process And Free Expansion
Text Solution
|
- Work done in the process C rarrA is
Text Solution
|
- Work done during isothermal volume change (in one step) under a consta...
Text Solution
|
- Statement-1 : Work done in isothermal reversible process is more than ...
Text Solution
|
- What is the work done in the free expansion of an ideal gas in reversi...
Text Solution
|
- निम्न में से कौन-सा प्रक्रम उत्क्रमणीय है और कौन-सा अनुत्क्रणीय ? (अ...
Text Solution
|
- Find work done in the irreversible process C to A.
Text Solution
|
- During expansion of a gas into vaccum (P("ext")=0), Work done is zero ...
Text Solution
|