A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
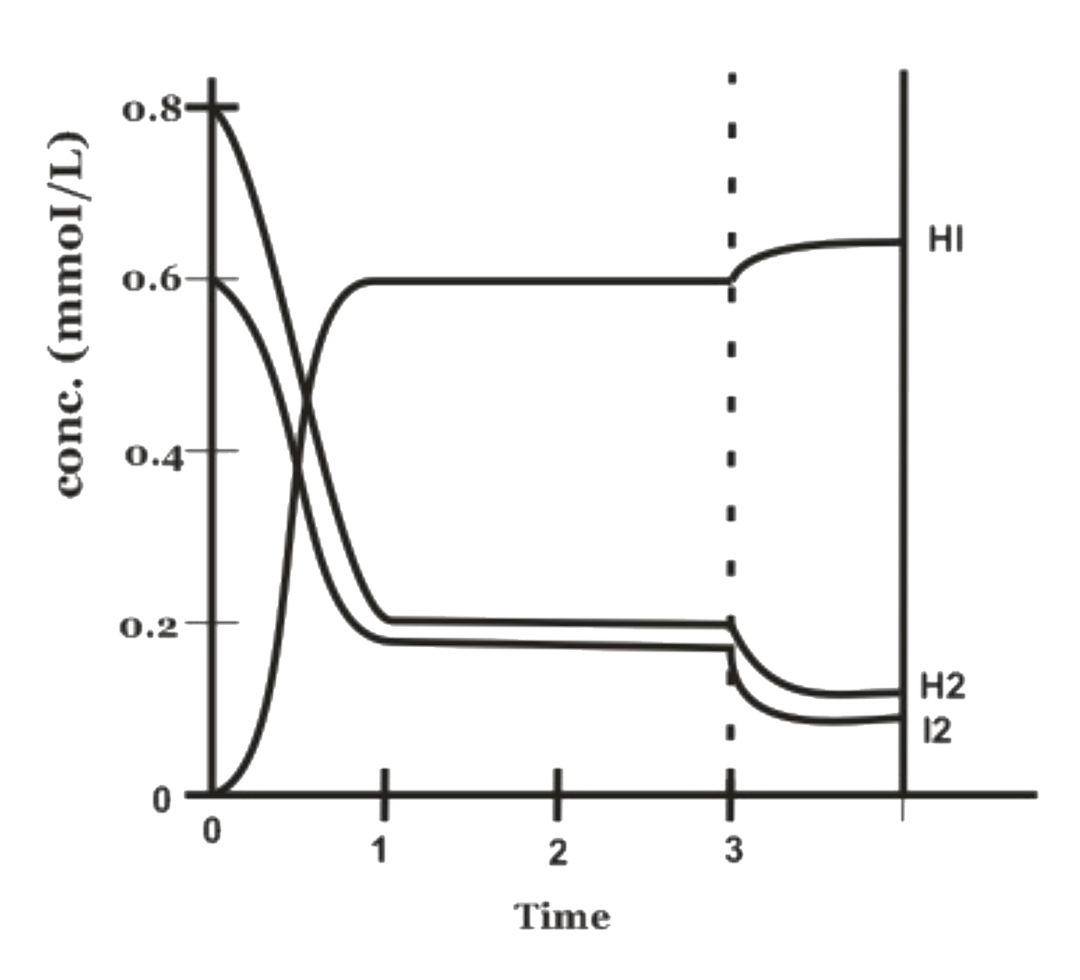
- The equation for the reaction in the figure below is : H2(g)+I2(g)+"...
Text Solution
|
- For the reversible reaction H2(g)+I2(g)hArr2HI(g) the value of the...
Text Solution
|
- If for homogeneous equilibrium, H2(g)+I2(g)hArr2HI(g), K(eq)=1 , then
Text Solution
|
- The equation for the reaction in the figure given below is AB(5)(g)+He...
Text Solution
|
- 1 mole each of H2(g)and I2(g) are introduced in a 1L evacuated vessel ...
Text Solution
|
- The value of equilibrium constant of the reaction. HI(g)hArr(1)/(2)H2(...
Text Solution
|
- The equation for the reaction in the figure below is : H2(g)+i2(g)+"...
Text Solution
|
- For the reaction H2(g) +I2(g) hArr 2HI(g), the equilibrium constant K...
Text Solution
|
- H(2)(g) + I(2)(g) rarr 2HI(g) , DeltaH= 12.40 kcal इस समीकरण के अनु...
Text Solution
|