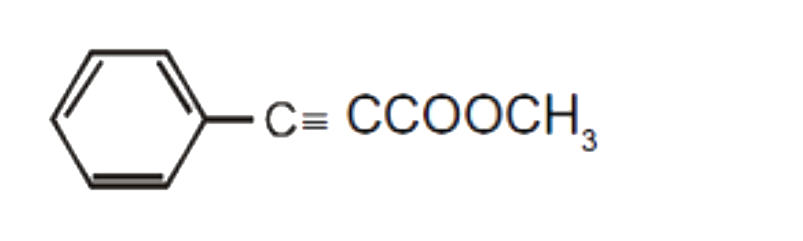A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the following molecule: What are the number of sigma and...
Text Solution
|
- Number of sigma and pi bonds present in sulphuric acid molecule is an...
Text Solution
|
- The number of sigma (sigma) and p i(pi) bonds present in a molecule of...
Text Solution
|
- What is the number of sigma (sigma) and pi (pi) bonds present in sulp...
Text Solution
|
- Write the number of sigma and pi bonds in the following molecules?
Text Solution
|
- The number of sigma bonds and pi bonds in CO(2) molecule are respecti...
Text Solution
|
- Consider the following molecule: What are the number of sigma and...
Text Solution
|
- What is the number of sigma and pi-bonds in a molecule of ethyne?
Text Solution
|
- What is the number of sigma (sigma) and pi (pi) bonds present in sulp...
Text Solution
|