A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The diagram shows the effect of an enzyme working in the human digest...
Text Solution
|
- During the process of digestion, the proteins present in food material...
Text Solution
|
- (a) What is the role of hydrochloric acid in our stomach ? (b) What is...
Text Solution
|
- The first enzyme that the food encounters in human digestive system is...
Text Solution
|
- In jejunum,enzyme required for digestion of amino acid containing comp...
Text Solution
|
- During the process of digestion, the proteins present in food material...
Text Solution
|
- During the process of digestion, the proteins present in food material...
Text Solution
|
- During the process of digestion, the proteins present in food material...
Text Solution
|
- During the process of digestion, the proteins present in food material...
Text Solution
|
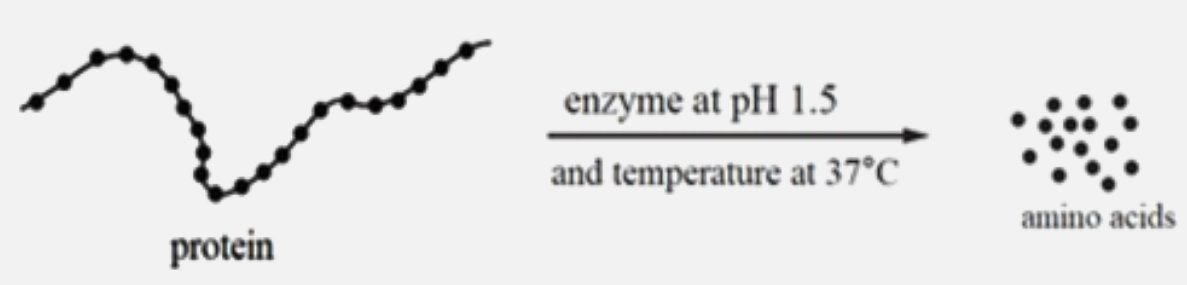 `underset("and temperature at" 37^@C)overset("enzyme at PH1.5)rarr`
`underset("and temperature at" 37^@C)overset("enzyme at PH1.5)rarr`