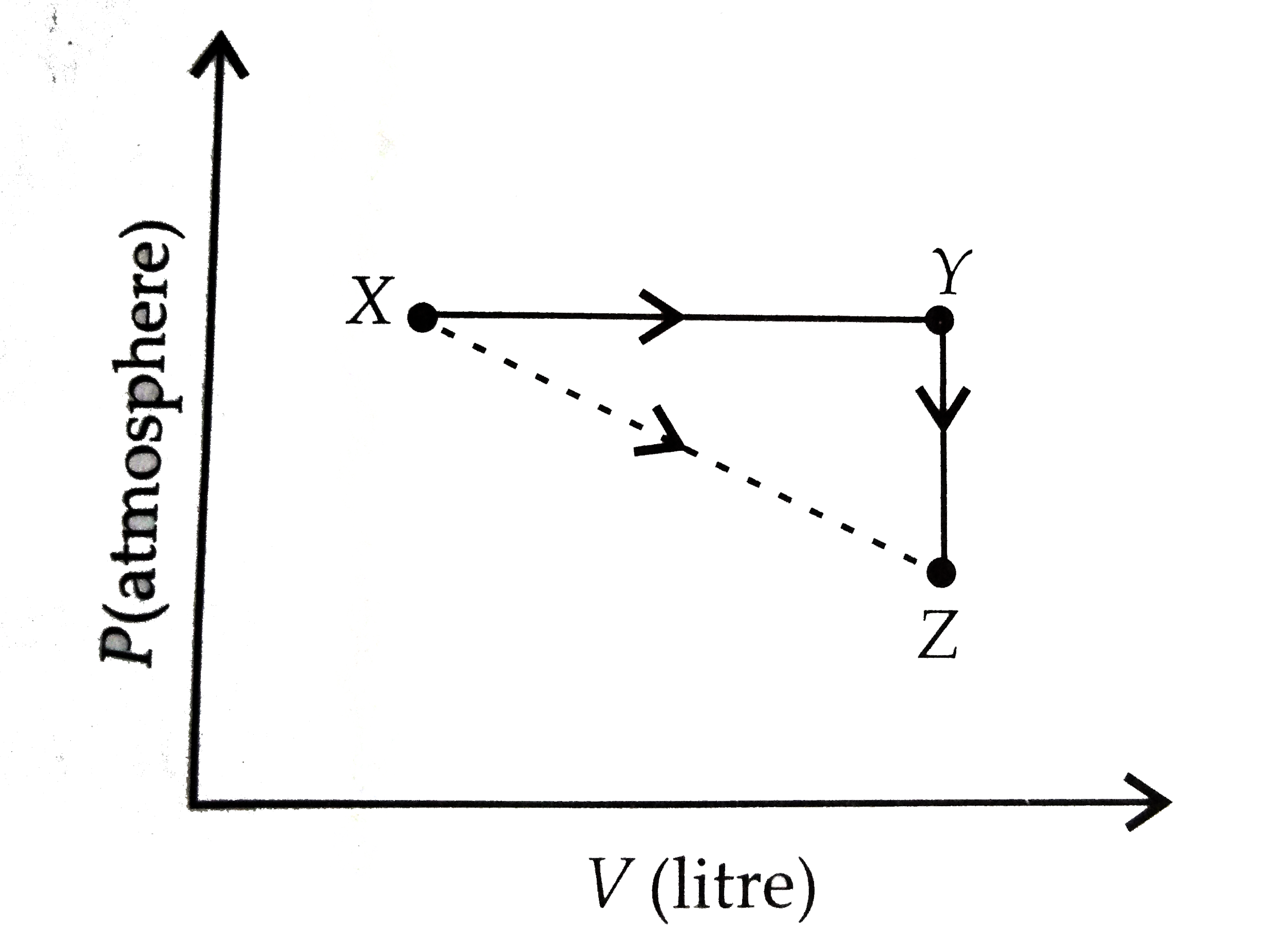
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For an ideal gas, consider only P-V work in going from an initial stat...
Text Solution
|
- The state of an ideal gas is changed through an isothermal process at ...
Text Solution
|
- For an ideal gas, consider only P-V work in going from an initial stat...
Text Solution
|
- An ideal gas undergoes change in its state from the initial state I to...
Text Solution
|
- P - V diagram of an ideal gas is shown. The gas undergoes from initial...
Text Solution
|
- एक आदर्श गैस के लिए आरम्भिक अवस्था x से अन्तिम अवस्था : तक जाने - के ल...
Text Solution
|
- In the given figure, an ideal gas changes its state from A to C by fou...
Text Solution
|
- एक आदर्श गैस के लिए, आरम्भिक अवस्था से अंतिम अवस्था Z तक जाने के लिए क...
Text Solution
|
- For an ideal gas, consider only P-V work in going from an initial stat...
Text Solution
|