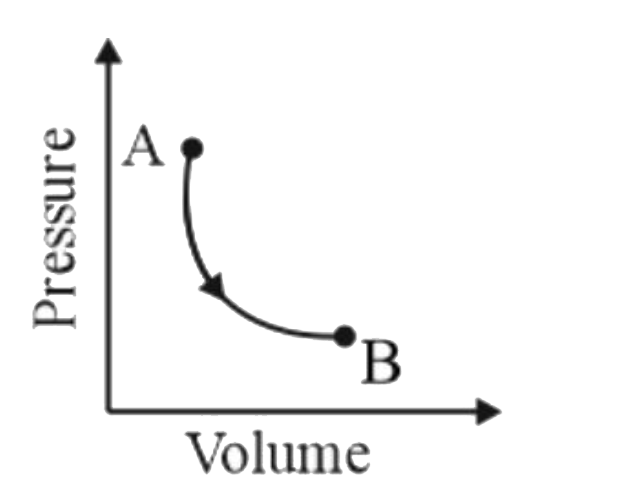
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The pressure and volume of an ideal gas are related as p alpha 1/v^2 ...
Text Solution
|
- The pressure p and volume V of an ideal gas both increase in a process...
Text Solution
|
- Pressure and volume of a gas changes from (p0V0) to (p0/4, 2V0) in a p...
Text Solution
|
- An ideal gas has initial volume V and pressure p. In doubling its volu...
Text Solution
|
- The pressure (P) and volume (V) of an ideal gas both increase in a pro...
Text Solution
|
- One mole of an ideal gas passes through a process where pressure and v...
Text Solution
|
- The pressure and volume of an ideal gas are related as p alpha 1/v^2 ...
Text Solution
|
- One mole of the ideal gas through the process p= p0 [ 1 - alpha ((V)...
Text Solution
|
- The variation of pressure P with volume V for an ideal monatomic gas d...
Text Solution
|