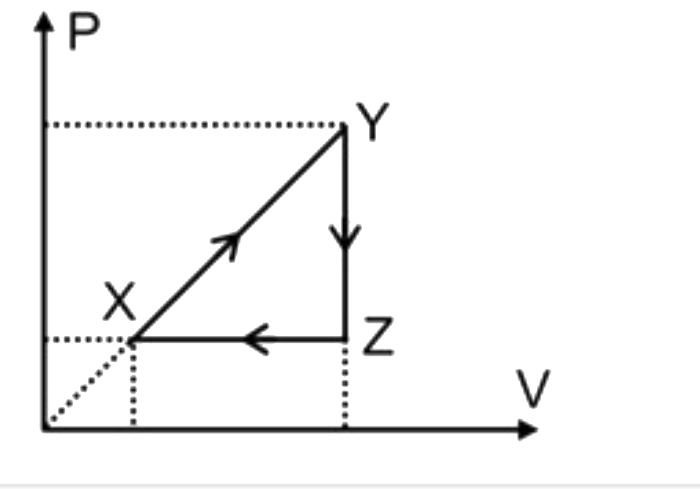
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In the show indicator diagram over pressure - volume scales 'n' moles ...
Text Solution
|
- A temperature T is measured by a constant volume gas thermometer (i) T...
Text Solution
|
- Figure illustrates a cycle conducted with n moles of an ideal gas. In ...
Text Solution
|
- The volume vs. temperature graph of 1 mole of an ideal gas is given be...
Text Solution
|
- आदर्श गैस मापक्रम पर न्यूनतम सम्भव ताप कितना होता है ?
Text Solution
|
- The number of molecules in an ideal gas with a volume of V at pressure...
Text Solution
|
- In the show indicator diagram over pressure - volume scales 'n' moles ...
Text Solution
|
- Two moles of a confined ideal monatomic gas begin at state A in the pr...
Text Solution
|
- A monatomic ideal gas is contained in a rigid container of volume V wi...
Text Solution
|