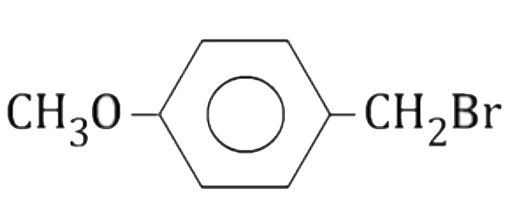
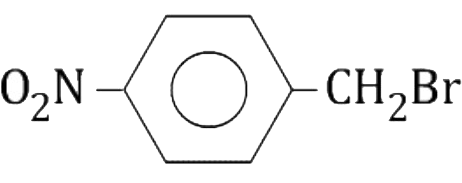
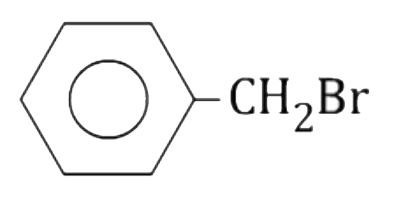
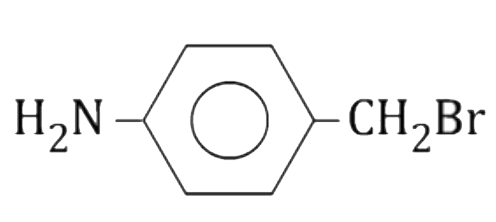
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which one of the following compounds undergoes predominantly SN^2 reac...
Text Solution
|
- Which of the following is polar aprotic solvent ?
Text Solution
|
- Which one of the following compounds undergoes predominantly SN^2 reac...
Text Solution
|
- Which one of the following compounds undergoes predominantly S(N^(2)) ...
Text Solution
|
- Assertion (A) : SN^(2) reaction is carried out in the presence of pola...
Text Solution
|
- Phenol on reaction with Br(2) in non-polar aprotic solvent furnishes
Text Solution
|
- How many of the following are polar aprotic solvents?
Text Solution
|
- Which one of the following compounds undergoes predominantly SN^2 reac...
Text Solution
|
- Which of the following is / are polar aprotic solvents
Text Solution
|