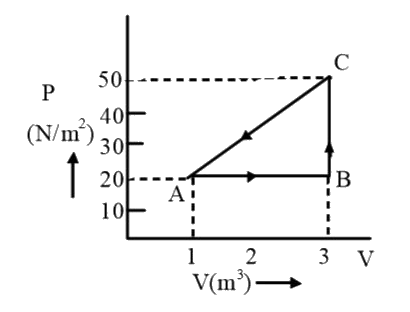
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In the diagram, the graph between volume and pressure for a thermodyna...
Text Solution
|
- A body is displaced from position A to position B. Kinetic and potenti...
Text Solution
|
- Let Delta U(1) and Delta U(2) be the changes in internal energy of an ...
Text Solution
|
- An ideal monoatomic gas is taken through the thermodynamic states AtoB...
Text Solution
|
- संलग्न चित्र में किसी ऊष्मागतिक निकाय का P-V आरेख प्रदर्शित किया गयाह...
Text Solution
|
- In the diagram, the graph between volume and pressure for a thermodyna...
Text Solution
|
- In the diagram, the graph between volume and pressure for a thermodyna...
Text Solution
|
- In a thermodynamic process, pressure of a fixed mass of a gas is chang...
Text Solution
|
- The internal energy of a system is U(1) . In a process, work done by t...
Text Solution
|