A
B
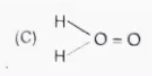
C
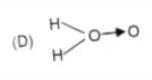
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following is a true structure of H(2)O(2) ?
Text Solution
|
- Which of the following statements about H(2)O(2) is not true ?
Text Solution
|
- The structure of H(2)O(2) is
Text Solution
|
- The structure of H(2)O(2) is
Text Solution
|
- The structure of H(2)O(2) is
Text Solution
|
- The structure of H(2)O(2) is
Text Solution
|
- The structure of H(2)O(2) is:
Text Solution
|
- Which of following true structure of F(2)O(2) :
Text Solution
|
- Which of the following is true for DeltaH H(2)O(l) hArr H(2)O(g)100^(@...
Text Solution
|