A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
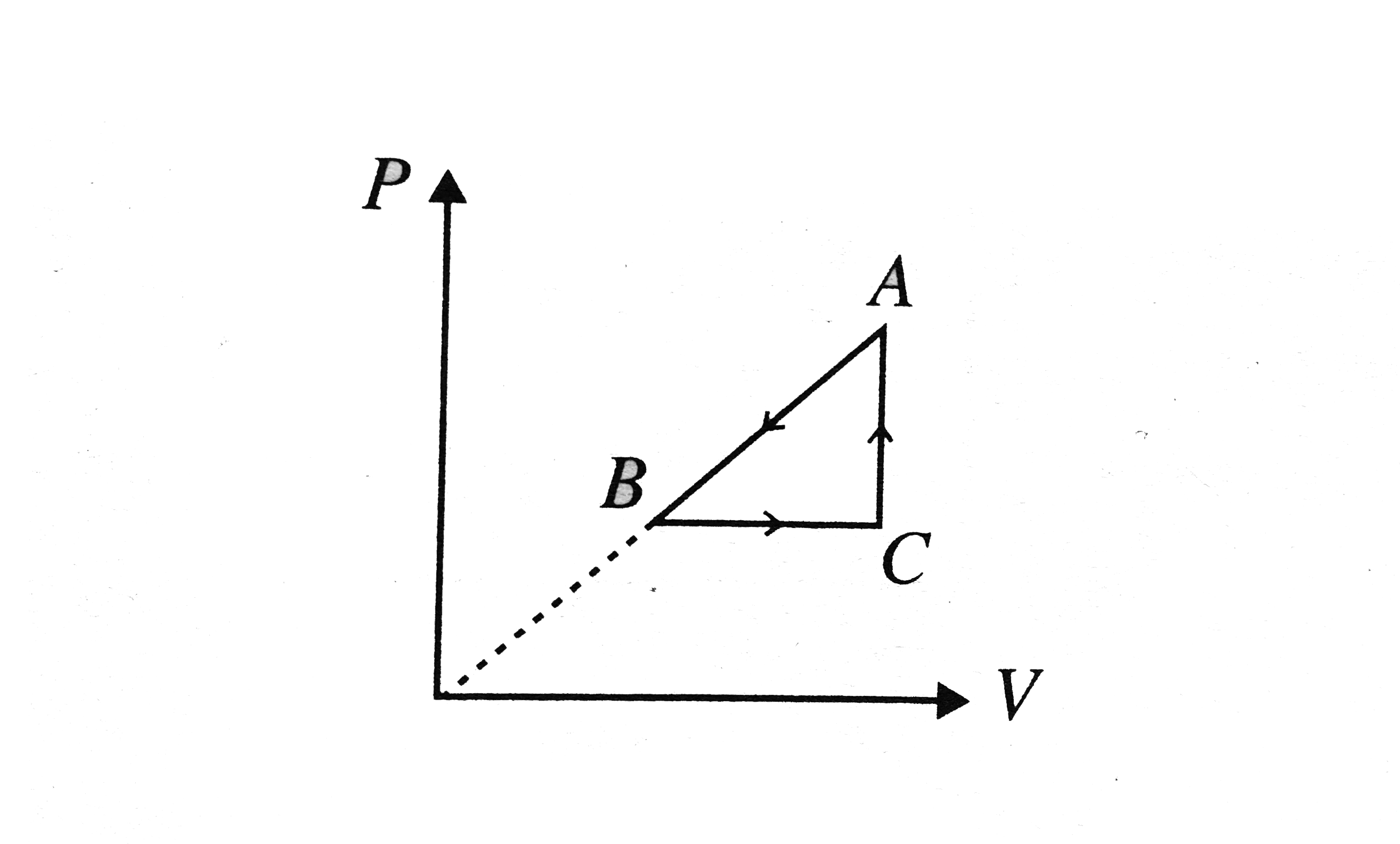
- P - V diagram of a cyclic process ABCA is as shown in Fig. Choose the ...
Text Solution
|
- If a ,b ,c are sides of a scalene triangle, then value of |a b c b c ...
Text Solution
|
- Which of the following is true during adsorption? a. DeltaG , DeltaH...
Text Solution
|
- A cyclic process ABCA is shown in the V-T diagram process on the P-V
Text Solution
|
- P - V diagram of a cyclic process ABCA is as shown in Fig. Choose the ...
Text Solution
|
- संलग्न चित्र में, किसी आदर्श गैस की ऊष्मागतिकीय प्रक्रियाओ का दाब- आयत...
Text Solution
|
- When Delta V is negative then
Text Solution
|
- One mole of an ideal gas is put through a series fo changes as shown b...
Text Solution
|
- নির্দিষ্ট ভরের কোনাে গ্যাসকে ABCA প্রক্রিয়ার মধ্য দিয়ে নিয়ে যাওয়া ...
Text Solution
|